Human muscle satellite cells as targets of Chikungunya virus infection - PubMed (original) (raw)
doi: 10.1371/journal.pone.0000527.
Michel Huerre, Jean-Pierre Riviere, Lark L Coffey, Philippe V Afonso, Vincent Mouly, Jean de Monredon, Jean-Christophe Roger, Mohamed El Amrani, Jean-Luc Yvin, Marie-Christine Jaffar, Marie-Pascale Frenkiel, Marion Sourisseau, Olivier Schwartz, Gillian Butler-Browne, Philippe Desprès, Antoine Gessain, Pierre-Emmanuel Ceccaldi
Affiliations
- PMID: 17565380
- PMCID: PMC1885285
- DOI: 10.1371/journal.pone.0000527
Human muscle satellite cells as targets of Chikungunya virus infection
Simona Ozden et al. PLoS One. 2007.
Abstract
Background: Chikungunya (CHIK) virus is a mosquito-transmitted alphavirus that causes in humans an acute infection characterised by fever, polyarthralgia, head-ache, and myalgia. Since 2005, the emergence of CHIK virus was associated with an unprecedented magnitude outbreak of CHIK disease in the Indian Ocean. Clinically, this outbreak was characterized by invalidating poly-arthralgia, with myalgia being reported in 97.7% of cases. Since the cellular targets of CHIK virus in humans are unknown, we studied the pathogenic events and targets of CHIK infection in skeletal muscle.
Methodology/principal findings: Immunohistology on muscle biopsies from two CHIK virus-infected patients with myositic syndrome showed that viral antigens were found exclusively inside skeletal muscle progenitor cells (designed as satelllite cells), and not in muscle fibers. To evaluate the ability of CHIK virus to replicate in human satellite cells, we assessed virus infection on primary human muscle cells; viral growth was observed in CHIK virus-infected satellite cells with a cytopathic effect, whereas myotubes were essentially refractory to infection.
Conclusions/significance: This report provides new insights into CHIK virus pathogenesis, since it is the first to identify a cellular target of CHIK virus in humans and to report a selective infection of muscle satellite cells by a viral agent in humans.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
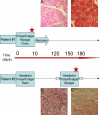
Figure 1. Time-course and histopathological data concerning CHIK virus-infected patients #1 and #2.
Patient #1, 67 year old, (from whom a quadriceps biopsy was obtained during the CHIK virus epidemic outbreak in the Reunion Island) presented a classical clinical picture of CHIK virus infection: polyarthtralgia, fever, and myalgia. In addition, signs of rhabdomyolysis were reported (creatine phosphokinase 41600 IU/mL). The muscle biopsy was performed on the quadriceps muscle, for pathological diagnosis during the acute phase of the illness (red star). During the following weeks, the patient recovered. Patient#2 is a 71 year old woman who complained in January, 2006, of headaches, arthralgia and a rash; around three months later, she was admitted to hospital with a classical clinical picture of CHIK virus infection, including fever, headaches, joint pain, and myalgia. A biopsy was performed in the quadriceps muscle during this recurrent phase of the disease (red star). 1A: A section from the muscle biopsy of patient #1. Hematoxylin-eosin staining. No important cellular infiltrates were observed. Atrophy and necrosis of muscle fibers could be seen, as well as central nuclei (arrow). (magnification: ×60). 1B: A section from the muscle biopsy of patient #1. Hematoxylin-eosin staining. Vacuolization of muscle fibers were detected. (magnification: ×140). 1C, D: Sections from the muscle biopsy of patient #2 showing an important mononuclear infiltration in transversal (C) and longitudinal sections (D) stained by hematoxylin-eosin (C) and Masson's trichrome (D); an important amount of fibrosis is also observed (D); (magnification:C: ×140; D: ×80)
Figure 2. Identification of infiltrating cells by immunocytochemistry.
Muscle sections from patient#1 (A to E)) or patient#2 (F to J) werre processed as described in material and methods for immunocytochemistry (immunoperoxydase detection) for the following antigens: CD56 (mouse monoclonal antibody; Dako; A,E); CD8 (mouse monoclonal antibody; Dako; B,G); CD20 (mouse monoclonal antibody; Dako; C,H); CD3 (Neomarker; D,I); CD68 (mouse monoclonal antibody; Dako; E,J). In patient#1 (A to E), the few infiltrating cells were mostly detected as immunoreactive for CD68 and CD3. In patient#2, the massive infiltrates were mainly composed of CD68 and CD3 immunoreactive cells. Counterstain: henatoxylin-eosin. Immunoreactive cells (red-brown color) are indicated by arrows, except in I and J (too numerous). Magnification: ×170.
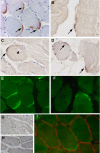
Figure 3. Immunocytochemical detection of CHIK virus antigens in a section of the muscle biopsy from patients #1 and #2.
Detection of CHIK virus antigens was performed on paraffin embedded sections (indirect immunofluorescence and immunoperoxydase) A–D: Detection of CHIK virus in muscle biopsy sections from patient #1 (immunoperoxydase) (Fig 1A: AEC as substrate; Fig. B,C,D: DAB as substrate). Immunoreactivity is detected on fusiform curved shaped cells located at the periphery of the myotubes, either as multiple cells per microscopic field (A,D), or as single cell per field (B,C). Some immunoreactive cells (arrows) were detected at the periphery of muscle fibers with central nuclei (C, arrowhead). E–F: Detection of CHIK virus in muscle biopsy sections from patient #1 (E) and #2 (F) using the immunofluorescence technique. Whereas the muscle biopsy of patient #1 numerous cells exhibited immunoreactivity (E), at the periphery of myotubes, as assessed by the (immunoperoxydase technique), very rare immunoreactive cells could be detected in sections of the muscle biopsy from patient #2 (F). G: West Nile virus (IS-98-ST1 srtrain) used as the primary antibody (muscle section from patient#1); no staining was observed; similar results were obtained with sera against yellow fever virus, or dengue type-1 virus (data not shown). G,H: sections from a non-infected patient (G) or from a HTLV-1-infected patient with myositic syndrome (H) used as controls; no significant immunoreactivity detected. I: double labeling by immunofluorescence of CHIK virus antigens (green staining; HMAFs anti CHIK virus #1) and laminin (red staining; rabbit anti-laminin polyclonal antibody). Note the CHIK virus immunoreactive cells are located beneath the basal lamina. (magnification: ×300(A,C,D,E,F); ×400(B); ×420 (I); ×100 (G,H)).
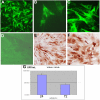
Figure 4. Infection of cultured human muscle satellite cells by CHIK virus.
Human muscle satellite cells were isolated from the quadriceps (surgical wasting)as previously described ; They were grown on coverslips and, once differentiated into mytubes or not, infected with two different CHIK virus isolates (isolates 05-115 and 06-049) or vehicle alone. For infection experiments, muscle cells (satellites and myotubes) were exposed for 2 h. at 37°C in medium containing 1% FCS to about 1 to 10 pfu/cell of CHIK virus isolates 06-049 and 05-115 . Cultures were kept for 24, 36, 48, and 72 h. (according to experiments) in Ham's F-10 medium supplemented with 2% fetal calf serum. Immunocytochemical analysis was performed as described in Fig. 2, both by indirect immunofluorescence or immunoperoxydase, after acetone fixation. A: Detection by immunofluorescence of CHIK virus antigens on satellite cells grown for 24 h. after CHIK virus infection (m.o.i. 10). HMAF anti-CHIK virus #1; (magnification:×250). B: Detection by immunofluorescence of CHIKV antigens on satellite cells grown for 24 h. after CHIK virus infection (m.o.i. 10), using a monoclonal antibody against alphavirus nucleocapsid. (magnification: ×1000). C: Detection by immunofluorescence of CHIK virus antigens on satellite cells grown for 48 h. after CHIK virus infection (m.o.i. 10). HMAF anti-CHIK virus #1; (magnification: ×400). D: Absence of infection of myotubes, 24 h. after CHIK virus infection (m.o.i. 10), using HMAF anti-CHIK virus as primary antibody. (magnification: ×200). E and F: Detection by immunoperoxydase of CHIK virus antigens on satellite cells (from two different donors) grown for 36 h. after CHIK virus infection (m.o.i. 10). HMAF anti-CHIK virus #1; (magnification: ×200(E), ×400(F)). G: viral yielding of satellite cells from donor #1 at 24 and 72 h. post-infection by CHIK virus. Analysis of viral yielding was performed by plaque titration on cultured mosquitoe cells as previously described and is expressed as UFF/mL.
Similar articles
- Differential diagnosis of Chikungunya, dengue viral infection and other acute febrile illnesses in children.
Laoprasopwattana K, Kaewjungwad L, Jarumanokul R, Geater A. Laoprasopwattana K, et al. Pediatr Infect Dis J. 2012 May;31(5):459-63. doi: 10.1097/INF.0b013e31824bb06d. Pediatr Infect Dis J. 2012. PMID: 22301475 - Chikungunya fever diagnosed among international travelers--United States, 2005-2006.
Centers for Disease Control and Prevention (CDC). Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep. 2006 Sep 29;55(38):1040-2. MMWR Morb Mortal Wkly Rep. 2006. PMID: 17008866 - Re-emergence of Chikungunya virus in Malaysia.
Kumarasamy V, Prathapa S, Zuridah H, Chem YK, Norizah I, Chua KB. Kumarasamy V, et al. Med J Malaysia. 2006 Jun;61(2):221-5. Med J Malaysia. 2006. PMID: 16898316 - Chikungunya: a paradigm of emergence and globalization of vector-borne diseases.
Simon F, Savini H, Parola P. Simon F, et al. Med Clin North Am. 2008 Nov;92(6):1323-43, ix. doi: 10.1016/j.mcna.2008.07.008. Med Clin North Am. 2008. PMID: 19061754 Review. - Arthritis after infection with Chikungunya virus.
Ali Ou Alla S, Combe B. Ali Ou Alla S, et al. Best Pract Res Clin Rheumatol. 2011 Jun;25(3):337-46. doi: 10.1016/j.berh.2011.03.005. Best Pract Res Clin Rheumatol. 2011. PMID: 22100284 Review.
Cited by
- Unraveling the complex interplay: immunopathology and immune evasion strategies of alphaviruses with emphasis on neurological implications.
de Oliveira Souza R, Duarte Júnior JWB, Della Casa VS, Santoro Rosa D, Renia L, Claser C. de Oliveira Souza R, et al. Front Cell Infect Microbiol. 2024 Aug 15;14:1421571. doi: 10.3389/fcimb.2024.1421571. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39211797 Free PMC article. Review. - Chikungunya and Mayaro Viruses Induce Chronic Skeletal Muscle Atrophy Triggered by Pro-Inflammatory and Oxidative Response.
da Silva MOL, Figueiredo CM, Neris RLS, Guimarães-Andrade IP, Gavino-Leopoldino D, Miler-da-Silva LL, Valença HDM, Ladislau L, de Lima CVF, Coccarelli FM, Benjamim CF, Assunção-Miranda I. da Silva MOL, et al. Int J Mol Sci. 2024 Aug 16;25(16):8909. doi: 10.3390/ijms25168909. Int J Mol Sci. 2024. PMID: 39201595 Free PMC article. - Pathogenicity and virulence of chikungunya virus.
Freppel W, Silva LA, Stapleford KA, Herrero LJ. Freppel W, et al. Virulence. 2024 Dec;15(1):2396484. doi: 10.1080/21505594.2024.2396484. Epub 2024 Sep 1. Virulence. 2024. PMID: 39193780 Free PMC article. Review. - Long chikungunya? An overview to immunopathology of persistent arthralgia.
Silveira-Freitas JEP, Campagnolo ML, Dos Santos Cortez M, de Melo FF, Zarpelon-Schutz AC, Teixeira KN. Silveira-Freitas JEP, et al. World J Virol. 2024 Jun 25;13(2):89985. doi: 10.5501/wjv.v13.i2.89985. World J Virol. 2024. PMID: 38984075 Free PMC article. Review. - Neuroinvasion of emerging and re-emerging arboviruses: A scoping review.
Srichawla BS, Manan MR, Kipkorir V, Dhali A, Diebel S, Sawant T, Zia S, Carrion-Alvarez D, Suteja RC, Nurani K, Găman MA. Srichawla BS, et al. SAGE Open Med. 2024 May 6;12:20503121241229847. doi: 10.1177/20503121241229847. eCollection 2024. SAGE Open Med. 2024. PMID: 38711470 Free PMC article. Review.
References
- Weaver SC, Frolov IV. Togaviruses. In: Mahy B, ter Meulen V, editors. Topley & Wilson's Microbiology & Microbial Infections; Virology. 10th ed. London: Hodder Arnold; 2005. pp. 1010–1024.
- Tesh RB. Arthritides caused by mosquito-borne viruses. Annu Rev Med. 1982;33:31–40. - PubMed
- Outbreak news. Chikungunya and dengue, south-west Indian Ocean. Wkly Epidemiol Rec. 2006;81:106–108. - PubMed
- Outbreak news. Chikungunya, India. Wkly Epidemiol Rec. 2006;81:409–410. - PubMed
- Pfeffer M, Loscher T. Cases of chikungunya imported into Europe. Euro Surveill. 2006;11:E060316 060312. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials