PPARalpha in atherosclerosis and inflammation - PubMed (original) (raw)
Review
PPARalpha in atherosclerosis and inflammation
Fokko Zandbergen et al. Biochim Biophys Acta. 2007 Aug.
Abstract
Peroxisome proliferator-activated receptor (PPAR)alpha is a nuclear receptor activated by natural ligands such as fatty acids as well as by synthetic ligands such as fibrates currently used to treat dyslipidemia. PPARalpha regulates the expression of genes encoding proteins that are involved in lipid metabolism, fatty acid oxidation, and glucose homeostasis, thereby improving markers for atherosclerosis and insulin resistance. In addition, PPARalpha exerts anti-inflammatory effects both in the vascular wall and the liver. Here we provide an overview of the mechanisms through which PPARalpha affects the initiation and progression of atherosclerosis, with emphasis on the modulation of atherosclerosis-associated inflammatory responses. PPARalpha activation interferes with early steps in atherosclerosis by reducing leukocyte adhesion to activated endothelial cells of the arterial vessel wall and inhibiting subsequent transendothelial leukocyte migration. In later stages of atherosclerosis, evidence suggests activation of PPARalpha inhibits the formation of macrophage foam cells by regulating expression of genes involved in reverse cholesterol transport, formation of reactive oxygen species (ROS), and associated lipoprotein oxidative modification among others. Furthermore, PPARalpha may increase the stability of atherosclerotic plaques and limit plaque thrombogenicity. These various effects may be linked to the generation of PPARalpha ligands by endogenous mechanisms of lipoprotein metabolism. In spite of this dataset, other reports implicate PPARalpha in responses such as hypertension and diabetic cardiomyopathy. Although some clinical trials data with fibrates suggest that fibrates may decrease cardiovascular events, other studies have been less clear, in terms of benefit. Independent of the clinical effects of currently used drugs purported to achieve PPARalpha, extensive data establish the importance of PPARalpha in the transcriptional regulation of lipid metabolism, atherosclerosis, and inflammation.
Figures
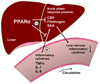
Figure 1. PPARα activation modulates the hepatic acute phase response
Hepatic expression of inflammatory cytokines – such as CRP, fibrinogen and SAA – is inhibited by PPARα, both under basal conditions and after stimulation with cytokines such as IL-1, IL-6 and TNFα from the circulation. CRP, C-reactive protein; IL-1, interleukin-1; IL-6, interleukin-6; SAA, serum amyloid A; TNFα, tumor necrosis factor α.
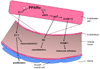
Figure 2. PPARα activation modulates reactivity of the vascular endothelium
Activation of PPARα in ECs decreases production and secretion of ET-1, which would be expected to decrease vasoconstriction and SMC proliferation. PPARα has been reported to increase levels of endothelial nitric oxide synthetase (eNOS), which is the enzyme responsible for NO generation. ENOS induction would be expected to increase NO production and thus increase vasodilatation. Increased NO decreases expression of VCAM-1, a key player in leukocyte adhesion to the endothelium. PPARα activation can directly decrease VCAM-1 expression, which is thought to involve inhibition of NFκB activity. eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; NO, nitric oxide; SMC, smooth muscle cell; VCAM-1, vascular cell adhesion molecule-1.
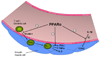
Figure 3. PPARα inhibits local inflammatory responses in the endothelium
PPARα activation in the vasculature inhibits adhesion of T-cells and dendritic cells to the endothelium through inhibition of VCAM-1 expression. PPARα activation also decreases expression of inflammatory cytokines such as IFN-γ, TNFα, IL-2 by Th1 cells. In addition, PPARα inhibits the induction of COX2 expression by SMCs activated in response to IL-1β. COX2, cyclooxygenase 2; IFN-γ, interferon- γ; IL-2, interleukin 2.
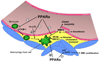
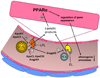
Figure 4. PPARα activation modulates atherosclerotic plaque formation at multiple levels
In addition to its effects on VCAM-1 expression, PPARα activation also purportedly limits macrophage foam cell formation by inhibiting oxidative modification of LDL and by promoting reverse cholesterol transport via increased SR-BI and ABCA1 expression. PPARα activation may also decrease plaque thrombogenicity by inhibiting expression of the procoagulant tissue factor (TF). PPARα activation may also repress expression of MMP9, an enzyme implicated in plaque rupture, and decrease SMC proliferation. MMP9, matrix metalloproteinase.
Figure 5. Specific pathways of lipoprotein metabolism activates PPARα
VLDL and HDL hydrolysis by LPL and EL respectively can generate PPARα activation. In response to the action of these enzymes on these lipoprotein substrates, PPARα target genes are regulated, including targets implicated in atherosclerosis, like VCAM-1. When these lipolytic pathways are integrated into PPAR responses, the possibility that other lipase regulators, like ApoCII and ApoAV, as well as LPL inhibitors like ApoCI, ApoCIII, Angptl3 and Angptl4 may also regulate PPAR activity becomes apparent. Angptl, angiopoietin-like protein; Apo, apoprotein; EL, endothelial lipase; HDL, high density lipoprotein; LPL, lipoprotein lipase; VLDL, very low density lipoprotein.
Similar articles
- Peroxisome Proliferator-Activated Receptor α in Lipid Metabolism and Atherosclerosis.
Yu XH, Zheng XL, Tang CK. Yu XH, et al. Adv Clin Chem. 2015;71:171-203. doi: 10.1016/bs.acc.2015.06.005. Epub 2015 Jul 23. Adv Clin Chem. 2015. PMID: 26411415 Review. - Peroxisome proliferator-activated receptor alpha (PPARalpha) and athero-sclerosis.
Gouni-Berthold I, Krone W. Gouni-Berthold I, et al. Curr Drug Targets Cardiovasc Haematol Disord. 2005 Dec;5(6):513-23. doi: 10.2174/156800605774962022. Curr Drug Targets Cardiovasc Haematol Disord. 2005. PMID: 16503871 Review. - Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-alpha activators: clinical and experimental evidence.
Zambon A, Gervois P, Pauletto P, Fruchart JC, Staels B. Zambon A, et al. Arterioscler Thromb Vasc Biol. 2006 May;26(5):977-86. doi: 10.1161/01.ATV.0000204327.96431.9a. Epub 2006 Jan 19. Arterioscler Thromb Vasc Biol. 2006. PMID: 16424352 Review. - Macrophage expression of peroxisome proliferator-activated receptor-alpha reduces atherosclerosis in low-density lipoprotein receptor-deficient mice.
Babaev VR, Ishiguro H, Ding L, Yancey PG, Dove DE, Kovacs WJ, Semenkovich CF, Fazio S, Linton MF. Babaev VR, et al. Circulation. 2007 Sep 18;116(12):1404-12. doi: 10.1161/CIRCULATIONAHA.106.684704. Epub 2007 Aug 27. Circulation. 2007. PMID: 17724261 - Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis.
Lefebvre P, Chinetti G, Fruchart JC, Staels B. Lefebvre P, et al. J Clin Invest. 2006 Mar;116(3):571-80. doi: 10.1172/JCI27989. J Clin Invest. 2006. PMID: 16511589 Free PMC article. Review.
Cited by
- Drug metabolizing enzyme induction pathways in experimental non-alcoholic steatohepatitis.
Fisher CD, Jackson JP, Lickteig AJ, Augustine LM, Cherrington NJ. Fisher CD, et al. Arch Toxicol. 2008 Dec;82(12):959-64. doi: 10.1007/s00204-008-0312-z. Epub 2008 May 17. Arch Toxicol. 2008. PMID: 18488193 Free PMC article. - Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism.
Cao D, Khan Z, Li X, Saito S, Bernstein EA, Victor AR, Ahmed F, Hoshi AO, Veiras LC, Shibata T, Che M, Cai L, Temel RE, Giani JF, Luthringer DJ, Divakaruni AS, Okwan-Duodu D, Bernstein KE. Cao D, et al. Cardiovasc Res. 2023 Aug 7;119(9):1825-1841. doi: 10.1093/cvr/cvad082. Cardiovasc Res. 2023. PMID: 37225143 Free PMC article. - Editor's Highlight: Neonatal Activation of the Xenobiotic-Sensors PXR and CAR Results in Acute and Persistent Down-regulation of PPARα-Signaling in Mouse Liver.
Li CY, Cheng SL, Bammler TK, Cui JY. Li CY, et al. Toxicol Sci. 2016 Oct;153(2):282-302. doi: 10.1093/toxsci/kfw127. Epub 2016 Jul 13. Toxicol Sci. 2016. PMID: 27413110 Free PMC article. - Vitamin C restores healthy aging in a mouse model for Werner syndrome.
Massip L, Garand C, Paquet ER, Cogger VC, O'Reilly JN, Tworek L, Hatherell A, Taylor CG, Thorin E, Zahradka P, Le Couteur DG, Lebel M. Massip L, et al. FASEB J. 2010 Jan;24(1):158-72. doi: 10.1096/fj.09-137133. Epub 2009 Sep 9. FASEB J. 2010. PMID: 19741171 Free PMC article. - Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation.
Fuentes-Antrás J, Ioan AM, Tuñón J, Egido J, Lorenzo O. Fuentes-Antrás J, et al. Int J Endocrinol. 2014;2014:847827. doi: 10.1155/2014/847827. Epub 2014 Mar 12. Int J Endocrinol. 2014. PMID: 24744784 Free PMC article. Review.
References
- McGovern PG, Pankow JS, Shahar E, Doliszny KM, Folsom AR, Blackburn H, Luepker RV. Recent trends in acute coronary heart disease--mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med. 1996;334:884–890. - PubMed
- Wilhelmsen L, Rosengren A, Johansson S, Lappas G. Coronary heart disease attack rate, incidence and mortality 1975–1994 in Goteborg, Sweden. Eur Heart J. 1997;18:572–581. - PubMed
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. - PubMed
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. - PubMed
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL048743/HL/NHLBI NIH HHS/United States
- P01 HL048743-13A15364/HL/NHLBI NIH HHS/United States
- R01 HL071745/HL/NHLBI NIH HHS/United States
- R01 HL071745-04/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical