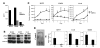Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas - PubMed (original) (raw)
Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas
Sizhi Paul Gao et al. J Clin Invest. 2007 Dec.
Abstract
Persistently activated or tyrosine-phosphorylated STAT3 (pSTAT3) is found in 50% of lung adenocarcinomas. pSTAT3 is found in primary adenocarcinomas and cell lines harboring somatic-activating mutations in the tyrosine kinase domain of EGFR. Treatment of cell lines with either an EGFR inhibitor or an src kinase inhibitor had no effect on pSTAT3 levels, whereas a pan-JAK inhibitor (P6) blocked activation of STAT3 and inhibited tumorigenesis. Cell lines expressing these persistently activated mutant EGFRs also produced high IL-6 levels, and blockade of the IL-6/gp130/JAK pathway led to a decrease in pSTAT3 levels. In addition, reduction of IL-6 levels by RNA interference led to a decrease in tumorigenesis. Introduction of persistently activated EGFR into immortalized breast epithelial cells led to tumorigenesis, IL-6 expression, and STAT3 activation, all of which could be inhibited with P6 or gp130 blockade. Furthermore, inhibition of EGFR activity in multiple cell lines partially blocked transcription of IL-6 and concurrently decreased production and release of IL-6. Finally, immunohistochemical analysis revealed a positive correlation between pSTAT3 and IL-6 positivity in primary lung adenocarcinomas. Therefore, mutant EGFR could activate the gp130/JAK/STAT3 pathway by means of IL-6 upregulation in primary human lung adenocarcinomas, making this pathway a potential target for cancer treatment.
Figures
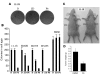
Figure 1. STAT3 is tyrosine phosphorylated in primary lung adenocarcinomas.
Immunohistochemical analysis of 92 primary lung adenocarcinomas for tyrosine-phosphorylated STAT3 was performed; 46 were scored as 0; 27 as +1; 16 as +2; and 3 as +3, with associated percentages indicated in parentheses. Two examples of each are shown. Original magnification, ×200.
Figure 2. JAK inhibition, but not EGFR inhibition, blocks STAT3 phosphorylation and induces growth arrest in human lung adenocarcinoma–derived cell lines harboring mutant EGFR.
(A) Extracts from H3255, H1975, H1650, 11-18, and H460 cell lines, treated with DMSO (D), ZD (5 μM), P6 (1 μM), or BMS (5 μM) for 16 hours, were analyzed for phospho- and total EGFR, STAT3, AKT, and MAPK as well as α-tubulin as a loading control by Western blot analysis. (B) Extracts from 11-18 cells, treated with DMSO, P6 (1 μM), or ZD (5 μM) for 30 minutes and stimulated with EGF (E) (100 ng/ml) for 10 minutes, were analyzed for pEGFR, EGFR, pSTAT3, STAT3, pMAPK, and MAPK. (C) Cell proliferation of H3255, H1975, H1650, 11-18, and H460 cells treated with P6 was determined over a 5-day period. Light gray line, DMSO treatment; black line, P6 treatment. (D) Extracts obtained from scrambled control (C) and STAT3 shRNA–expressing (S3Sh-expressing) H1650 cells were analyzed for pSTAT3, STAT3, and α-tubulin (left). Proliferation of the H1650 control (light gray line) and H1650 S3Sh (black line) was evaluated by calcein AM.
Figure 3. P6 inhibits tumorigenesis of human lung adenocarcinoma cell lines in vitro and in vivo.
(A) 11-18, H1650, H1975, H3255, and H460 cells (5 × 103/well) were plated in soft agar in the presence of DMSO, ZD, or P6. Colony numbers were counted after 14 days. A representative colony growth of 11-18 is shown with the indicated treatment. (B) Soft agar colony numbers of treated 11-18, H1650, H1975, H3255, and H460 cell lines are shown (mean ± SD). (C) 11-18 cells were treated with DMSO or P6 for 16 hours and injected into the flanks of nude mice. Size and weight of tumors were determined after 14 days (mean ± SD). Examples of 2 animals with representative injections are shown. (D) Weight of the tumors from DMSO- or P6-treated cells are shown (mean ± SD).
Figure 4. The IL-6/gp130/JAK pathway mediates STAT3 phosphorylation in lung adenocarcinoma–derived cell lines.
(A) Extracts isolated from H3255, 11-18, and H1650 cell lines treated with control mouse IgG, gp130-blocking mAb (B-R3), or IL-6–blocking mAb (αIL-6) after a medium change were analyzed by Western blotting for pSTAT3, STAT3, and α-tubulin as a loading control. (B) CM collected from 11-18 cells was added to MCF-10A cells in the presence of control mouse IgG, B-R3, αIL-6, IL-6R (αIL-6R), OSM (αOSM), and LIF (αLIF) blocking antibodies. Extracts isolated from these treated cells were analyzed by Western blot analysis for pSTAT3 and STAT3.
Figure 5. Blockade of IL-6 signaling with IL-6 shRNA inhibits growth of cell lines.
(A) IL-6 shRNA lentivirus (ShRNA) and control lentivirus (C) were introduced into H1975, H1650, and 11-18 cell lines. After 72 hours of selection with puromycin, levels of IL-6 were determined by ELISA of CM. (B) Extracts isolated from the above-described cell lines were analyzed by Western blotting for pSTAT3, STAT3, and α-tubulin as a loading control. (C) A total of 2,000 cells/cm2 were seeded, and proliferation was determined daily with the use of calcein AM. (D) H1975, 11-18, and H1650 cells, expressing either control or IL-6 shRNA (Sh), were injected into the flanks of nude mice. The tumor weight was determined after 21 days (mean ± SD) (right). An example of an animal injected with H1975 cells infected with control or IL-6 shRNA is shown (left).
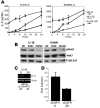
Figure 6. Overexpression of ΔEGFR protein in MCF-10A cells induces persistent phosphorylation of STAT3, AKT, and MAPK as well as tumorigenesis.
(A) Extracts isolated from control pBabe (pB) and ΔEGFR-expressing MCF-10A cells were analyzed by Western blot for phospho- and total EGFR, STAT3, AKT, and MAPK as well as α-tubulin as a loading control. The lanes were run on the same gel but were noncontiguous. (B) Soft agar colony formation assays for MCF-10A control pB and ΔEGFR-expressing cells are shown. Colony numbers are shown below. (C) MCF-10A control pB and ΔEGFR-expressing cells were injected into the flanks of nude mice. No tumor was detected with pB, while ΔEGFR-expressing MCF-10A cells formed tumors. Tumor weight was determined after 21 days (mean ± SD) (right). An example of an animal injected with MCF-10A–ΔEGFR–expressing cells is shown (left).
Figure 7. Overexpression of ΔEGFR mediates IL-6 production of MCF-10A cells.
(A) Extracts isolated from ΔEGFR MCF-10A cells treated with DMSO, ZD, P6, B-R3, or αIL-6 for 16 hours were analyzed by Western blotting for pEGFR, EGFR, pSTAT3, STAT3, pAKT, pMAPK, and MAPK. The last lane was run on the same gel but was noncontiguous. (B) Human IL-6 mRNA levels from MCF-10A control (pB) and ΔEGFR MCF-10A cells were determined by RT-PCR and normalized to β_-actin_. (C) Extracts were isolated from MCF-10A cells treated with CM from control cells (pB) and ΔEGFR MCF-10A cells and analyzed by Western blotting for pSTAT3 and STAT3.
Figure 8. EGFR tyrosine kinase inhibition reduces de novo production of IL-6.
(A) 11-18 and H1650 cells were treated with a medium change (MC) with ZD (MC+ZD), no MC with ZD (No MC+ZD), or MC with DMSO (MC+D). Levels of IL-6 were measured by ELISA at the indicated times after the addition of ZD (mean ± SD). (B) Shown are extracts isolated from 11-18 and H1650 cells treated as described above after 16 hours and analyzed for pSTAT3, STAT3, and α-tubulin. (C) Human IL-6 mRNA levels from 11-18 cells treated with DMSO or ZD for 16 hours after a medium change were determined by RT-PCR and normalized to β_-actin_. The same mRNA samples were analyzed by quantitative real-time PCR (QPCR), and the IL-6 mRNA levels (normalized to hypoxanthine-guanine phosphoribosyltransferase [_HPRT_]) are shown below. (D) NIH3T3 cells were cotransfected with an IL-6 reporter construct, a TK-Renilla construct (for transfection/loading control), and either a pB vector (baseline activity) or a pB-ΔEGFR expression construct. Twenty-four hours after transfection, DMSO or ZD was added, and an additional 24 hours later, cells were lysed and subjected to firefly and Renilla luciferase activity measurements. The bars show fold induction over the baseline activity (mean ± SD).
Figure 9. pSTAT3 levels correlate positively with IL-6 expression in primary lung adenocarcinomas.
Immunohistochemical analysis of TMAs of primary lung adenocarcinomas (92 tumor specimens) for IL-6 were scored as 0 (5/92), +1 (26/92), +2 (54/92), or +3 (7/92). Examples of selected tumor specimens from sequential sections of the same TMAs stained for IL-6 (top, left to right, 0, +1, +3, and +3) and pSTAT3 (bottom, left to right, 0, 0, +3, and +3) are shown. A positive correlation was observed in specimens that were pSTAT3 positive (+1, +2, or +3) and expressing moderate-to-high (+2 to +3) levels of IL-6 as determined by analysis with the Fisher exact test (P < 0.001).
Comment in
- IL-6 involvement in epithelial cancers.
Schafer ZT, Brugge JS. Schafer ZT, et al. J Clin Invest. 2007 Dec;117(12):3660-3. doi: 10.1172/JCI34237. J Clin Invest. 2007. PMID: 18060028 Free PMC article.
Similar articles
- IL-6 involvement in epithelial cancers.
Schafer ZT, Brugge JS. Schafer ZT, et al. J Clin Invest. 2007 Dec;117(12):3660-3. doi: 10.1172/JCI34237. J Clin Invest. 2007. PMID: 18060028 Free PMC article. - EGFR and KRAS mutations do not enrich for the activation of IL-6, JAK1 or phosphorylated STAT3 in resected lung adenocarcinoma.
Clay TD, Russell PA, Do H, Sundararajan V, Conron M, Wright GM, Solomon B, Dobrovic A, McLachlan SA, Moore MM. Clay TD, et al. Med Oncol. 2017 Sep 6;34(10):175. doi: 10.1007/s12032-017-1031-1. Med Oncol. 2017. PMID: 28879441 - Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer.
Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, Gerald WL, Bornmann W, Bromberg JF. Berishaj M, et al. Breast Cancer Res. 2007;9(3):R32. doi: 10.1186/bcr1680. Breast Cancer Res. 2007. PMID: 17531096 Free PMC article. - Targeting the IL-6/JAK/STAT3 signalling axis in cancer.
Johnson DE, O'Keefe RA, Grandis JR. Johnson DE, et al. Nat Rev Clin Oncol. 2018 Apr;15(4):234-248. doi: 10.1038/nrclinonc.2018.8. Epub 2018 Feb 6. Nat Rev Clin Oncol. 2018. PMID: 29405201 Free PMC article. Review. - Roads to Stat3 Paved with Cadherins.
Adan H, Daniel J, Raptis L. Adan H, et al. Cells. 2022 Aug 16;11(16):2537. doi: 10.3390/cells11162537. Cells. 2022. PMID: 36010614 Free PMC article. Review.
Cited by
- IL-8 signaling is involved in resistance of lung carcinoma cells to erlotinib.
Fernando RI, Hamilton DH, Dominguez C, David JM, McCampbell KK, Palena C. Fernando RI, et al. Oncotarget. 2016 Jul 5;7(27):42031-42044. doi: 10.18632/oncotarget.9662. Oncotarget. 2016. PMID: 27248176 Free PMC article. - The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis.
Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, Cotari J, Alpaugh ML, de Stanchina E, Manova K, Li M, Bonafe M, Ceccarelli C, Taffurelli M, Santini D, Altan-Bonnet G, Kaplan R, Norton L, Nishimoto N, Huszar D, Lyden D, Bromberg J. Chang Q, et al. Neoplasia. 2013 Jul;15(7):848-62. doi: 10.1593/neo.13706. Neoplasia. 2013. PMID: 23814496 Free PMC article. - Regulation of histone deacetylase activities and functions by phosphorylation and its physiological relevance.
Bahl S, Seto E. Bahl S, et al. Cell Mol Life Sci. 2021 Jan;78(2):427-445. doi: 10.1007/s00018-020-03599-4. Epub 2020 Jul 18. Cell Mol Life Sci. 2021. PMID: 32683534 Free PMC article. Review. - Differential interleukin-6/Stat3 signaling as a function of cellular context mediates Ras-induced transformation.
Leslie K, Gao SP, Berishaj M, Podsypanina K, Ho H, Ivashkiv L, Bromberg J. Leslie K, et al. Breast Cancer Res. 2010;12(5):R80. doi: 10.1186/bcr2725. Epub 2010 Oct 7. Breast Cancer Res. 2010. PMID: 20929542 Free PMC article. - The Role of MRE11 in the IL-6/STAT3 Pathway of Lung Cancer Cells.
Wu CY, Shu LH, Liu HT, Cheng YC, Wu YH, Wu YH. Wu CY, et al. Curr Issues Mol Biol. 2022 Dec 5;44(12):6132-6144. doi: 10.3390/cimb44120418. Curr Issues Mol Biol. 2022. PMID: 36547079 Free PMC article.
References
- Jett J.R., Midthun D.E. Screening for lung cancer: current status and future directions: Thomas A. Neff lecture. Chest. 2004;125:158S–162S. - PubMed
- Hayes D.N., et al. Gene expression profiling reveals reproducible human lung adenocarcinoma subtypes in multiple independent patient cohorts. J. Clin. Oncol. 2006;24:5079–5090. - PubMed
- Lynch T.J., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. - PubMed
- Paez J.G., et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
- Riely G.J., Politi K.A., Miller V.A., Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin. Cancer Res. 2006;12:7232–7241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous