Lysyl oxidase propeptide inhibits smooth muscle cell signaling and proliferation - PubMed (original) (raw)
Lysyl oxidase propeptide inhibits smooth muscle cell signaling and proliferation
Paola A Hurtado et al. Biochem Biophys Res Commun. 2008.
Abstract
Lysyl oxidase is required for the normal biosynthesis and maturation of collagen and elastin. It is expressed by vascular smooth muscle cells, and its increased expression has been previously found in atherosclerosis and in models of balloon angioplasty. The lysyl oxidase propeptide (LOX-PP) has more recently been found to have biological activity as a tumor suppressor, and it inhibits Erk1/2 Map kinase activation. We reasoned that LOX-PP may have functions in normal non-transformed cells. We, therefore, investigated its effects on smooth muscle cells, focusing on important biological processes mediated by Erk1/2-dependent signaling pathways including proliferation and matrix metalloproteinase-9 (MMP-9) expression. In addition, we investigated whether evidence for accumulation of LOX-PP could be found in vivo in a femoral artery injury model. Recombinant LOX-PP was expressed and purified, and was found to inhibit primary rat aorta smooth muscle cell proliferation and DNA synthesis by more than 50%. TNF-alpha-stimulated MMP-9 expression and Erk1/2 activation were both significantly inhibited by LOX-PP. Immunohistochemistry studies carried out with affinity purified anti-LOX-PP antibody showed that LOX-PP epitopes were expressed at elevated levels in vascular lesions of injured arteries. These novel data suggest that LOX-PP may provide a feedback control mechanism that serves to inhibit properties associated with the development of vascular pathology.
Figures
Figure 1
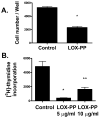
LOX-PP inhibits growth of SMC in cultures. In A, primary neonatal rat aortic SMCs were grown as described in the presence of LOX-PP (10 μg/ml) or vehicle and the number of viable cells per culture determined on day 2 n = 3; *, p < 0.0001). In B, Rat aortic SMCs were cultured, LOX-PP or vehicle was added to cultures for 24 hours. Tritiated thymidine incorporation was then determined. Values shown are the averages of 3 independent cultures (*, p < 0.005; **, p < 0.05).
Figure 2
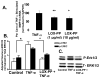
LOX-PP inhibits (A) TNF-α stimulation of MMP-9 mRNA levels, and (B and C) Erk1/2 activation. In A, primary neonatal rat aortic SMCs were treated with or without 20 ng/ml TNF-α in the presence and absence of LOX-PP. MMP-9 and GAPDH mRNA levels were determined by qPCR with Taqman reagents. Data are pooled from two different experiments with three independent cultures each, and are expressed as a per cent of TNF-α-stimulated MMP-9 mRNA levels +/− SE, normalized to GAPDH. *, p<0.05; **, p<0.004, n=3. Primary neonatal rat aortic SMCs were cultured for 24 h in the presence or absence of 10 μg/ml LOX-PP or vehicle. Cells were then stimulated with 20 ng/ml TNF-α or vehicle (PBS) for 15 minutes. Cells were extracted and aliquots subjected to Western blotting for phosphorylated, and total Erk1/2, respectively. Panel (B) shows densitometric quantitation of phosphorylated Erk 1 and Erk 2 levels normalized to total Erk 1 and 2. *, p<0.05, n=3. Panel (C) contains a representative Western blot.
Figure 3
LOX-PP directly inhibits MEK2 activity, and not Erk2. Recombinant active MEK2 ( 1 ng) was incubated with 6.7 μg of inactive Erk2 in the presence or absence of recombinant LOX-PP purified from a bacterial expression system [LOX-PP (b)] [9] or from a mammalian expression system [LOX-PP (m)] at 30o C for 15 min. This incubation is stage I (Stg. I). Aliquots of Stage I reactions were then assayed for the activity of Erk2 by measuring the phosphorylation of myelin basic protein in the presence of [γ32P]-ATP [Stage II reaction (Stg. II)]. Some reactions contained LOX-PP only in the Stage II reaction. Data are means +/− SD of triplicate assays, and are from a representative experiment done three times with similar results (*, p<0.05).
Figure 4
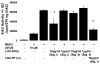
LOX-PP epitopes occur at elevated levels in guidewire-injured femoral arteries. Eleven week-old mice were subjected to guidewire-induced femoral artery injury or sham surgery as described [11, 25] and sacrificed after 14 days. Sections were subjected to immunohistochemistry with anti-LOX-PP antibody. Arrows mark positive signals in smooth muscle cells. Micrographs are from (A) sham-, and (B) injured-, femoral arteries (bar = 10 μm; n = 4); (L, lumen; N, neointima). (C) Western blot of a cultured neonatal rat aorta smooth muscle cell extract assayed with the LOX-PP antibody showing pro-lysyl oxidase (50 kDa), glycosylated LOX-PP (35 kDa) [7], and non-glycosylated LOX-PP (20 kDa) [9].
Similar articles
- Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts.
Vora SR, Palamakumbura AH, Mitsi M, Guo Y, Pischon N, Nugent MA, Trackman PC. Vora SR, et al. J Biol Chem. 2010 Mar 5;285(10):7384-93. doi: 10.1074/jbc.M109.033597. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048148 Free PMC article. - Lysyl oxidase propeptide promotes adipogenesis through inhibition of FGF-2 signaling.
Griner JD, Rogers CJ, Zhu MJ, Du M. Griner JD, et al. Adipocyte. 2017 Jan 2;6(1):12-19. doi: 10.1080/21623945.2016.1271511. Epub 2016 Dec 14. Adipocyte. 2017. PMID: 28452589 Free PMC article. - Lysyl oxidase enhances elastin synthesis and matrix formation by vascular smooth muscle cells.
Kothapalli CR, Ramamurthi A. Kothapalli CR, et al. J Tissue Eng Regen Med. 2009 Dec;3(8):655-61. doi: 10.1002/term.214. J Tissue Eng Regen Med. 2009. PMID: 19813219 Free PMC article. - Functions and Mechanisms of Pro-Lysyl Oxidase Processing in Cancers and Eye Pathologies with a Focus on Diabetic Retinopathy.
Trackman PC, Peymanfar Y, Roy S. Trackman PC, et al. Int J Mol Sci. 2022 May 3;23(9):5088. doi: 10.3390/ijms23095088. Int J Mol Sci. 2022. PMID: 35563478 Free PMC article. Review. - Lysyl Oxidase (LOX): Functional Contributions to Signaling Pathways.
Laczko R, Csiszar K. Laczko R, et al. Biomolecules. 2020 Jul 22;10(8):1093. doi: 10.3390/biom10081093. Biomolecules. 2020. PMID: 32708046 Free PMC article. Review.
Cited by
- Recombinant lysyl oxidase propeptide protein inhibits growth and promotes apoptosis of pre-existing murine breast cancer xenografts.
Bais MV, Nugent MA, Stephens DN, Sume SS, Kirsch KH, Sonenshein GE, Trackman PC. Bais MV, et al. PLoS One. 2012;7(2):e31188. doi: 10.1371/journal.pone.0031188. Epub 2012 Feb 8. PLoS One. 2012. PMID: 22363577 Free PMC article. - Vascular extracellular matrix and arterial mechanics.
Wagenseil JE, Mecham RP. Wagenseil JE, et al. Physiol Rev. 2009 Jul;89(3):957-89. doi: 10.1152/physrev.00041.2008. Physiol Rev. 2009. PMID: 19584318 Free PMC article. Review. - Lysyl oxidase-like-2 (LOXL2) is a major isoform in chondrocytes and is critically required for differentiation.
Iftikhar M, Hurtado P, Bais MV, Wigner N, Stephens DN, Gerstenfeld LC, Trackman PC. Iftikhar M, et al. J Biol Chem. 2011 Jan 14;286(2):909-18. doi: 10.1074/jbc.M110.155622. Epub 2010 Nov 11. J Biol Chem. 2011. PMID: 21071451 Free PMC article. - Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts.
Vora SR, Palamakumbura AH, Mitsi M, Guo Y, Pischon N, Nugent MA, Trackman PC. Vora SR, et al. J Biol Chem. 2010 Mar 5;285(10):7384-93. doi: 10.1074/jbc.M109.033597. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048148 Free PMC article. - The lysyl oxidase pro-peptide attenuates fibronectin-mediated activation of focal adhesion kinase and p130Cas in breast cancer cells.
Zhao Y, Min C, Vora SR, Trackman PC, Sonenshein GE, Kirsch KH. Zhao Y, et al. J Biol Chem. 2009 Jan 16;284(3):1385-93. doi: 10.1074/jbc.M802612200. Epub 2008 Nov 21. J Biol Chem. 2009. PMID: 19029090 Free PMC article.
References
- Black SA, Jr, Palamakumbura AH, Stan M, Trackman PC. Tissue-specific mechanisms for CCN2/CTGF persistence in fibrotic gingiva: interactions between cAMP and MAPK signaling pathways, and prostaglandin E2-EP3 receptor mediated activation of the c-JUN N-terminal kinase. J Biol Chem. 2007;282:15416–29. - PMC - PubMed
- Brasselet C, Durand E, Addad F, Al Haj Zen A, Smeets MB, Laurent-Maquin D, Bouthors S, Bellon G, de Kleijn D, Godeau G, Garnotel R, Gogly B, Lafont A. Collagen and elastin cross-linking: a mechanism of constrictive remodeling after arterial injury. Am J Physiol Heart Circ Physiol. 2005;289:H2228–33. - PubMed
- Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91:845–51. - PubMed
- Contente S, Kenyon K, Rimoldi D, Friedman RM. Expression of gene rrg is associated with reversion of NIH 3T3 transformed by LTR-c-H-ras. Science. 1990;249:796–8. - PubMed
- Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL013262/HL/NHLBI NIH HHS/United States
- DE11004/DE/NIDCR NIH HHS/United States
- R29 DE011004/DE/NIDCR NIH HHS/United States
- HL13262/HL/NHLBI NIH HHS/United States
- R01 CA082742/CA/NCI NIH HHS/United States
- CA082742/CA/NCI NIH HHS/United States
- R01 DE014066/DE/NIDCR NIH HHS/United States
- DE14066/DE/NIDCR NIH HHS/United States
- R01 DE011004/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous