Vesicle-independent extracellular release of a proinflammatory outer membrane lipoprotein in free-soluble form - PubMed (original) (raw)
Vesicle-independent extracellular release of a proinflammatory outer membrane lipoprotein in free-soluble form
Maribasappa Karched et al. BMC Microbiol. 2008.
Abstract
Background: Aggregatibacter actinomycetemcomitans is an oral bacterium associated with aggressively progressing periodontitis. Extracellular release of bacterial outer membrane proteins has been suggested to mainly occur via outer membrane vesicles. This study investigated the presence and conservation of peptidoglycan-associated lipoprotein (AaPAL) among A. actinomycetemcomitans strains, the immunostimulatory effect of AaPAL, and whether live cells release this structural outer membrane lipoprotein in free-soluble form independent of vesicles.
Results: The pal locus and its gene product were confirmed in clinical A. actinomycetemcomitans strains by PCR-restriction fragment length polymorphism and immunoblotting. Culturing under different growth conditions revealed no apparent requirement for the AaPAL expression. Inactivation of pal in a wild-type strain (D7S) and in its spontaneous laboratory variant (D7SS) resulted in pleiotropic cellular effects. In a cell culture insert model (filter pore size 0.02 mum), AaPAL was detected from filtrates when strains D7S and D7SS were incubated in serum or broth in the inserts. Electron microscopy showed that A. actinomycetemcomitans vesicles (0.05-0.2 mum) were larger than the filter pores and that there were no vesicles in the filtrates. The filtrates were immunoblot negative for a cytoplasmic marker, cyclic AMP (cAMP) receptor protein. An ex vivo model indicated cytokine production from human whole blood stimulated by AaPAL.
Conclusion: Free-soluble AaPAL can be extracellularly released in a process independent of vesicles.
Figures
Figure 1
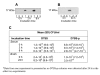
PCR-RFLP of pal and detection of its gene product, PAL, from clonally diverse A. actinomycetemcomitans strains. Panel A: Agarose gel electrophoresis of the PCR products shows amplicons with the expected size of pal (425 bp) for each strain. Panels B and C: Agarose gel electrophoresis of the purified PCR amplicons digested with _Dde_I and _Bsp_MI, separately. Panel D: immunoblot analysis of the A. actinomycetemcomitans whole cell protein preparations using anti-AaPAL peptide antiserum shows the expected 17-kDa signal for each strain. Lanes 1 through 12 strain identification (serotype; genotype): ATCC 29523 (a; 1), SA5002 (a; 1), ATCC 43718 (b; 2), SA5003 (b; 8), ATCC 33384 (c; 3), SA5005 (c; 3), SA5001 (d; 5), SA5007 (d; 22), SA5008 (e; 6), SA5011 (e; 20), CU1000R (f; nd*), SA5022 (f; 19) and standards (S). *nd: not determined.
Figure 2
AaPAL expression in different growth conditions of A. actinomycetemcomitans. Strains D7S (wild-type, lane 1), D7SS (spontaneous smooth-colony variant of D7S, lane 2), and D7SS-p (_pal_-deficient mutant of D7SS, lane 3) were cultured in various nutritional and atmospheric conditions (for details, see Materials and Methods). Bacterial whole cell protein preparations (10 μg/well) were subjected to immunoblot analysis with anti-AaPAL peptide antiserum. The OMP preparations from the strains D7SS (lane 4) and D7SS-p (lane 5) were used as a positive and a negative control, respectively. CO2: CO2-enriched air (5%); BA: blood agar; TSA: trypticase soy agar.
Figure 3
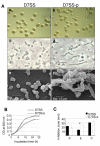
Role of AaPAL in cell physiology and membrane integrity. Panel A: Colony density and morphology (a, b) and the cell morphology of A. actinomycetemcomitans strains D7SS and D7SS-p grown on TSA as examined by phase contrast microscopy (c, d) and scanning electron microscopy (e, f). Panel B: Growth rates of D7SS and D7SS-p in TSB measured by turbidimetry (OD600). The results are shown from two separate experiments. Panel C: Antibiotic susceptibility comparison between the strains D7SS and D7SS-p. The results are means (SD) from three independent experiments (*P < 0.05). P, penicillin; E, erythromycin; C, cefotaxime.
Figure 4
Immunoblot detection of PAL release from A. actinomycetemcomitans cells exposed to heat-inactivated FCS or cultured in broth. The samples were obtained from cell-culture wells outside the inserts, where the bacteria [D7SS (lane 1) and D7SS-p (lane 2)] or No bacteria (lane 3) were incubated in serum for 2 h and 8 h (Panel A) or cultured in broth for 24 h (Panel B). AaPAL was detected by immunoblot using anti-AaPAL peptide antiserum. Samples for bacterial culture and enumeration were taken from the inserts containing serum or broth (Panel C) at the same time points as the samples for the immunoblots in Panels A and B. The results show means (SD) from three independent experiments.
Figure 5
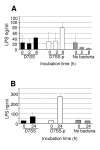
LPS released by A. actinomycetemcomitans cells using a cell culture insert model. Filtrates from serum incubation at 0, 2, and 8 h (Panel A) and broth culture at 0 and 24 h (Panel B) were subjected to quantification of LPS by Limulus assay. The results are means (SD) from two independent experiments.
Figure 6
Immunoblot analysis of the filtrates for the detection of a cytoplasmic marker, cAMP receptor protein. The whole cell protein preparations of _V. cholerae crp_+ strain (lane 1), _V. cholerae crp_- strain (lane 2) and A. actinomycetemcomitans D7SS (lane 3) were used as controls in the immunoblot with antibodies against V. cholerae cAMP receptor protein. Serum filtrates (8 h): D7SS (lane 4), D7SS-p (lane 5) and No bacteria control (lane 6). Broth filtrates (24 h): D7SS (lane 7), D7SS-p (lane 8) and No bacteria control (lane 9). Detection of cAMP receptor protein from precipitated filtrates of lysed (lane 10) and unlysed D7SS cells (lane 11) incubated in the inserts containing broth for 8 h.
Figure 7
Immunoblot analysis of the vesicle preparations from A. actinomycetemcomitans strains D7SS and D7SS-p. The samples (5 μg protein each) were subjected to immunoblot analysis using anti-AaPAL peptide antiserum (1:500). The chemiluminescence signal was captured using a CCD camera integrated in ChemiDoc™ XRS gel documentation system (Bio-Rad). The images captured by the camera were acquired and imported to the attached computer using QuantityOne® software (Bio-Rad). Lanes: D7SS (lanes 1, 3, 5) and D7SS-p (lanes 2, 4, 6). WCP; whole cell protein.
Figure 8
Electron micrographs of the vesicle preparations from filtrates. Filtrates from experiments studying in vitro AaPAL release into serum were subjected to vesicle preparation and subsequent electron microscopy. The filtrates were: A. actinomycetemcomitans D7SS at 8 h (Panel A), D7SS-p at 8 h (Panel B), No bacteria control at 2 h (Panel C). The vesicle preparation from A. actinomycetemcomitans D7SS whole cells (positive control) shows vesicles of different sizes indicated by arrows (Panel D). Bars; 0.2 μm.
Figure 9
Cytokine induction of human whole blood by AaPAL. A cytokine antibody array was used to detect cytokines produced by human whole blood after stimulation with purified AaPAL (5 and 1 μg/ml of blood) for 8 h. Storage buffer of AaPAL served as the negative control. Abbreviations: pos = positive, neg = negative.
Similar articles
- Immunoproteomics of Actinobacillus actinomycetemcomitans outer-membrane proteins reveal a highly immunoreactive peptidoglycan-associated lipoprotein.
Paul-Satyaseela M, Karched M, Bian Z, Ihalin R, Borén T, Arnqvist A, Chen C, Asikainen S. Paul-Satyaseela M, et al. J Med Microbiol. 2006 Jul;55(Pt 7):931-942. doi: 10.1099/jmm.0.46470-0. J Med Microbiol. 2006. PMID: 16772422 - Proinflammatory effect in whole blood by free soluble bacterial components released from planktonic and biofilm cells.
Oscarsson J, Karched M, Thay B, Chen C, Asikainen S. Oscarsson J, et al. BMC Microbiol. 2008 Nov 27;8:206. doi: 10.1186/1471-2180-8-206. BMC Microbiol. 2008. PMID: 19038023 Free PMC article. - Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin.
Kato S, Kowashi Y, Demuth DR. Kato S, et al. Microb Pathog. 2002 Jan;32(1):1-13. doi: 10.1006/mpat.2001.0474. Microb Pathog. 2002. PMID: 11782116 - Characteristics of Actinobacillus actinomycetemcomitans invasion of and adhesion to cultured epithelial cells.
Fives-Taylor P, Meyer D, Mintz K. Fives-Taylor P, et al. Adv Dent Res. 1995 Feb;9(1):55-62. doi: 10.1177/08959374950090011001. Adv Dent Res. 1995. PMID: 7669215 Review. - Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans.
Henderson B, Nair SP, Ward JM, Wilson M. Henderson B, et al. Annu Rev Microbiol. 2003;57:29-55. doi: 10.1146/annurev.micro.57.030502.090908. Annu Rev Microbiol. 2003. PMID: 14527274 Review.
Cited by
- Proteomics of extracellular vesicles produced by Granulicatella adiacens, which causes infective endocarditis.
Alkandari SA, Bhardwaj RG, Ellepola A, Karched M. Alkandari SA, et al. PLoS One. 2020 Nov 20;15(11):e0227657. doi: 10.1371/journal.pone.0227657. eCollection 2020. PLoS One. 2020. PMID: 33216751 Free PMC article. - Peptidoglycan-associated lipoprotein of Aggregatibacter actinomycetemcomitans induces apoptosis and production of proinflammatory cytokines via TLR2 in murine macrophages RAW 264.7 in vitro.
Ihalin R, Eneslätt K, Asikainen S. Ihalin R, et al. J Oral Microbiol. 2018 Mar 6;10(1):1442079. doi: 10.1080/20002297.2018.1442079. eCollection 2018. J Oral Microbiol. 2018. PMID: 29686780 Free PMC article. - Bacterial symbionts in oral niche use type VI secretion nanomachinery for fitness increase against pathobionts.
Oscarsson J, Bao K, Shiratsuchi A, Grossmann J, Wolski W, Aung KM, Lindholm M, Johansson A, Mowsumi FR, Wai SN, Belibasakis GN, Bostanci N. Oscarsson J, et al. iScience. 2024 Mar 29;27(5):109650. doi: 10.1016/j.isci.2024.109650. eCollection 2024 May 17. iScience. 2024. PMID: 38650989 Free PMC article. - Reductions in bacterial viability stimulate the production of Extra-intestinal Pathogenic Escherichia coli (ExPEC) cytoplasm-carrying Extracellular Vesicles (EVs).
Jiang M, Wang Z, Xia F, Wen Z, Chen R, Zhu D, Wang M, Zhuge X, Dai J. Jiang M, et al. PLoS Pathog. 2022 Oct 19;18(10):e1010908. doi: 10.1371/journal.ppat.1010908. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36260637 Free PMC article. - Flagella-dependent inhibition of biofilm formation by sub-inhibitory concentration of polymyxin B in Vibrio cholerae.
Giacomucci S, Cros CD, Perron X, Mathieu-Denoncourt A, Duperthuy M. Giacomucci S, et al. PLoS One. 2019 Aug 20;14(8):e0221431. doi: 10.1371/journal.pone.0221431. eCollection 2019. PLoS One. 2019. PMID: 31430343 Free PMC article.
References
- Norskov-Lauritsen N, Kilian M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov. and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates . Int J Syst Evol Microbiol. 2006;56:2135–2146. doi: 10.1099/ijs.0.64207-0. - DOI - PubMed
- Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. - PubMed
- Paul-Satyaseela M, Karched M, Bian Z, Ihalin R, Boren T, Arnqvist A, Chen C, Asikainen S. Immunoproteomics of Actinobacillus actinomycetemcomitans outer-membrane proteins reveal a highly immunoreactive peptidoglycan-associated lipoprotein . J Med Microbiol. 2006;55:931–942. doi: 10.1099/jmm.0.46470-0. - DOI - PubMed
- Sturgis JN. Organisation and evolution of the tol-pal gene cluster. J Mol Microbiol Biotechnol. 2001;3:113–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources