Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway - PubMed (original) (raw)
Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway
Núria de la Iglesia et al. Genes Dev. 2008.
Abstract
Activation of the transcription factor STAT3 is thought to potently promote oncogenesis in a variety of tissues, leading to intense efforts to develop STAT3 inhibitors for many tumors, including the highly malignant brain tumor glioblastoma. However, the function of STAT3 in glioblastoma pathogenesis has remained unknown. Here, we report that STAT3 plays a pro-oncogenic or tumor-suppressive role depending on the mutational profile of the tumor. Deficiency of the tumor suppressor PTEN triggers a cascade that inhibits STAT3 signaling in murine astrocytes and human glioblastoma tumors. Specifically, we forge a direct link between the PTEN-Akt-FOXO axis and the leukemia inhibitory factor receptor beta (LIFRbeta)-STAT3 signaling pathway. Accordingly, PTEN knockdown induces efficient malignant transformation of astrocytes upon knockout of the STAT3 gene. Remarkably, in contrast to the tumor-suppressive function of STAT3 in the PTEN pathway, STAT3 forms a complex with the oncoprotein epidermal growth factor receptor type III variant (EGFRvIII) in the nucleus and thereby mediates EGFRvIII-induced glial transformation. These findings indicate that STAT3 plays opposing roles in glial transformation depending on the genetic background of the tumor, providing the rationale for tailored therapeutic intervention in glioblastoma.
Figures
Figure 1.
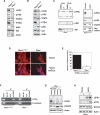
PTEN deficiency suppresses the LIFRβ–STAT3 signaling pathway. (A) Lysates of mouse Ptenwt/wt or _Pten_−/− astrocytes were immunoblotted with antibodies to LIFRβ, Tyr705-phosphorylated STAT3 (pYS3), total STAT3, PTEN, total Akt, Ser473-phosphorylated Akt (pAkt), or actin. (B) Immunoblotting of parental PtenloxP/loxP and _Pten_−/− astrocytes with antibodies used in A. Loss of PTEN expression was associated with an increase in phospho-Akt levels and with a decrease in LIFRβ and pYS3. Actin served as loading control. (C, left panel) Immunoblotting of lysates of _Pten_−/− astrocytes treated with the PI3K inhibitor LY294002 or DMSO vehicle control for 48 h. Asterisk indicates a nonspecific band. (Right panel) Immunoblotting of lysates from serum-starved PtenloxP/loxP astrocytes stably expressing a constitutively active form of Akt (caAkt). Activated Akt reduced LIFRβ and pYS3 levels. (D) Immunocytochemical analysis of PtenloxP/loxP and _Pten_−/− astrocytes with a FOXO3 antibody. Nuclei were stained with a DNA dye (Hoechst). FOXO3 was excluded from the nucleus in _Pten_−/− astrocytes. (E) PtenloxP/loxP and _Pten_−/− astrocytes were transfected with a luciferase reporter gene controlled by FOXO-binding sites (FHRE-Luc), together with a renilla expression plasmid to serve as an internal control, and subjected to a dual luciferase assay. FOXO-dependent transcription was reduced in _Pten_−/− astrocytes. (F) ChIP analysis at the endogenous LIFRβ promoter in PtenloxP/loxP and _Pten_−/− astrocytes with a FOXO3 antibody. A rabbit anti-HA antibody was used as negative control. The analysis was done with two independent sets of primers for the LIFRβ promoter. Negative controls for the PCR reaction were performed with primers for the E-cadherin promoter. Endogenous FOXO3 occupied the endogenous LIFRβ promoter in PtenloxP/loxP but not in _Pten_−/− astrocytes. (G) Immunoblotting with the LIFRβ and FOXO3 antibodies of PtenloxP/loxP and _Pten_−/− astrocytes infected with a FOXO3 RNAi-encoding lentivirus (FOXOi) or an empty vector and selected with puromycin. Actin served as loading control. Knockdown of endogenous FOXO3 reproducibly led to down-regulation of LIFRβ. (H) Immunoblotting of lysates of serum-starved _Pten_−/− astrocytes transfected with a LIFRβ expression plasmid or a control vector that were left untreated (−) or treated with LIF (L) for 15 min. LIFRβ restored LIF-induced STAT3 phosphorylation in _Pten_−/− astrocytes.
Figure 2.
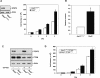
STAT3 knockout promotes astrocyte cell proliferation and invasiveness. (A, left panel) Immunoblotting of Stat3loxP/loxP or _Stat3_−/− astrocytes with an antibody to STAT3. Actin served as control for loading. (Right panel) Cell population growth of Stat3loxP/loxP and _Stat3_−/− astrocytes. STAT3 loss significantly increased astrocyte cell population growth rate (representative experiment of three independent experiments performed in triplicate; ANOVA; [*] P < 0.05; [**] P < 0.0001). (B) Quantification of the invasive potential of _Stat3_−/− astrocytes through a matrigel substrate. Stat3loxP/loxP and _Stat3_−/− astrocytes were seeded on top of an 8-μm pore size insert coated with matrigel and allowed to invade through the matrigel matrix for 22 h. Knockout of STAT3 significantly increased astrocyte cell invasiveness (n = 3; _t_-test; [*] P < 0.01). The effect of STAT3 loss on invasiveness was not secondary to a change in cell proliferation, as the invasive potential of these cells was measured at a time (22 h after plating) prior to a significant increase in cell number upon STAT3 knockout. (C) Immunoblotting of STAT3 and PTEN in Stat3loxP/loxP or _Stat3_−/− astrocytes that were uninfected or infected with a retrovirus encoding a shRNA directed against PTEN (shPTEN). Actin served as control for loading. (D) Cell population growth of PTEN knockdown (PTENshRNA) astrocytes. STAT3 loss significantly increased cell number of PTEN knockdown astrocytes (representative experiment of two independent experiments performed in triplicate; ANOVA; [*] P < 0.0001).
Figure 3.
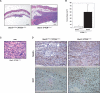
STAT3 suppresses PTEN deficiency-induced malignant cell transformation. (A,B) Stat3loxP/loxP;PTENshRNA or _Stat3_−/−;PTENshRNA astrocytes were injected subcutaneously into SCID mice. Eight weeks after injection, tumors were removed, measured, and stained. STAT3 loss induced an increase in tumor size as revealed by hematoxylin and eosin (H&E) staining (A) and tumor size measurements (n = 8; _t_-test, [*] P < 0.05) (B). Arrows in A show the tumor limits. Bar, 1 mm. (C) Histologic analysis of the _Stat3_−/−;PTENshRNA tumors by H&E staining. The tumors showed histologic features of neoplastic transformation including nuclear atypia, pleomorphism, and frequent mitotic figures (arrowheads). Bar, 100 μm. (D) Nestin and Ki67 immunostaining of Stat3loxP/loxP;PTENshRNA and _Stat3_−/−;PTENshRNA tumors. These tumors express nestin, a characteristic marker of glial tumors. Cell proliferation rate, as measured by the percentage of Ki67-positive cells, was higher in _Stat3_−/−;PTENshRNA tumors as compared with Stat3loxP/loxP;PTENshRNA tumors (64% vs. 25%, respectively; average of two tumors each). Bar, 100 μm.
Figure 4.
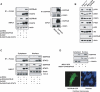
STAT3 interacts with EGFRvIII in the nucleus. (A, left panel) Lysates of 293T cells transfected with expression plasmids encoding Flag-STAT3, EGFRvIII, or both were immunprecipitated with a Flag antibody followed by immunoblotting with an EGFR or STAT3 antibody. Total lysates (Input) were also immunoblotted with these antibodies. Actin served as loading control. EGFRvIII interacted efficiently with STAT3. (Right panel) Lysates of stable MSCV-GFP and MSCV-GFP/EGFRvIII astrocytes were immunoprecipitated with an EGFRvIII antibody followed by immunoblotting with an EGFR or STAT3 antibody. Total lysates (Input) were also immunoblotted with these antibodies. EGFRvIII interacted with endogenous STAT3 in astrocytes. (B) Immunoblotting of PtenloxP/loxP and _Pten_−/− astrocytes, or stable MSCV-GFP and MSCV-GFP/EGFRvIII astrocytes with antibodies that recognize Tyr705-phosphorylated STAT3 (pYS3), Ser727-phosphorylated STAT3 (pSS3), total STAT3, PTEN, Ser473-phosphorylated Akt (pAkt), total Akt, and EGFR. Actin was used as loading control. EGFRvIII expression had little effect on STAT3 Tyr705 and Ser727 phosphorylation. (C) Lysates of 293T cells transfected with Flag-STAT3, EGFRvIII, or both were fractionated into cytoplasmic and nuclear fractions and then subjected to immunoprecipitation and immunoblotting analyses as in A. SnoN and 14–3–3 were used as nuclear or cytosolic markers, respectively. EGFRvIII interacted with STAT3 more efficiently in the nuclear than in the cytoplasmic fraction. (D) EGFRvIII is present in the nucleus of astrocytes. (Top panel) Lysates of EGFRvIII-expressing and control astrocytes were subjected to subcellular fractionation and immunoblotting with the EGFR antibody. Lamin A and α-tubulin were used as nuclear or cytosolic markers, respectively. (Bottom panel) Immortalized astrocytes transfected with a plasmid encoding an EGFRvIII-GFP fusion protein were fixed and imaged by confocal microscopy to detect GFP fluorescence. Hoechst was used to visualize nuclei. Arrowheads indicate the position of the nucleus.
Figure 5.
STAT3 mediates EGFRvIII-induced cell transformation. (A) Immunoblotting of EGFRvIII-expressing or control astrocytes with antibodies against EGFR and STAT3. Actin was used as loading control. (B) Cell population growth curves of Stat3loxP/loxP; EGFRvIII and _Stat3_−/−;EGFRvIII astrocytes. STAT3 knockout significantly decreased cell number (representative experiment of three independent experiments performed in triplicate; ANOVA; [*] P < 0.0001). (C) Size of EGFRvIII-expressing tumors. Stat3loxP/loxP; EGFRvIII and _Stat3_−/−;EGFRvIII astrocytes were injected subcutaneously into SCID mice. Four weeks after injection, tumors were excised, measured, and stained. Tumors were only present in Stat3loxP/loxP;EGFRvIII-injected mice (n = 6; _t_-test; [*] P < 0.05). (D) H&E staining of Stat3loxP/loxP;EGFRvIII tumors confirmed the presence of tumor cells. Mitotic figures are indicated by arrowheads. Bar, 100 μm. (E, left panel) Immunoblotting of PTENshRNA;EGFRvIII astrocytes with EGFR and STAT3 antibodies. Actin served as loading control. (Right panel) Size of PTENshRNA;EGFRvIII-expressing tumors. PTENshRNA;EGFRvIII astrocytes were injected subcutaneously into SCID mice. Four weeks after injection, tumors were excised and measured. Tumors were only present in Stat3loxP/loxP; PTENshRNA;EGFRvIII-injected mice (n = 4; _t_-test; [*] P < 0.001).
Figure 6.
STAT3 signaling in human glioblastoma specimens. (A) Immunoblotting of lysates of human glioblastoma samples (GBM) with antibodies to EGFR that recognize both wild-type and EGFRvIII forms, PTEN, LIFRβ, Tyr705-phosphorylated STAT3 (pYS3), or total STAT3. GAPDH was used as loading control. (B,C) Spearman correlation matrix of PTEN and EGFRvIII levels (B) or PTEN, LIFRβ, and pYS3 levels (C) measured as continuous variables in the immunoblots shown in A. The Spearman rank correlation coefficients, rs ([*] P < 0.05; [**] P < 0.005; [***] P < 0.0001), are shown.
Figure 7.
Dual role of STAT3 in tumorigenesis. (A) A PTEN-regulated STAT3 tumor-suppressive pathway. (B) EGFRvIII induces an oncogenic switch in STAT3 function.
Similar articles
- STAT3-iNOS Signaling Mediates EGFRvIII-Induced Glial Proliferation and Transformation.
Puram SV, Yeung CM, Jahani-Asl A, Lin C, de la Iglesia N, Konopka G, Jackson-Grusby L, Bonni A. Puram SV, et al. J Neurosci. 2012 Jun 6;32(23):7806-18. doi: 10.1523/JNEUROSCI.3243-11.2012. J Neurosci. 2012. PMID: 22674257 Free PMC article. - EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts.
Lal B, Goodwin CR, Sang Y, Foss CA, Cornet K, Muzamil S, Pomper MG, Kim J, Laterra J. Lal B, et al. Mol Cancer Ther. 2009 Jul;8(7):1751-60. doi: 10.1158/1535-7163.MCT-09-0188. Epub 2009 Jul 7. Mol Cancer Ther. 2009. PMID: 19584231 Free PMC article. - On-target JAK2/STAT3 inhibition slows disease progression in orthotopic xenografts of human glioblastoma brain tumor stem cells.
Stechishin OD, Luchman HA, Ruan Y, Blough MD, Nguyen SA, Kelly JJ, Cairncross JG, Weiss S. Stechishin OD, et al. Neuro Oncol. 2013 Feb;15(2):198-207. doi: 10.1093/neuonc/nos302. Epub 2012 Dec 21. Neuro Oncol. 2013. PMID: 23262510 Free PMC article. - STAT3 regulation of glioblastoma pathogenesis.
de la Iglesia N, Puram SV, Bonni A. de la Iglesia N, et al. Curr Mol Med. 2009 Jun;9(5):580-90. doi: 10.2174/156652409788488739. Curr Mol Med. 2009. PMID: 19601808 Free PMC article. Review. - PTEN signaling pathways in glioblastoma.
Koul D. Koul D. Cancer Biol Ther. 2008 Sep;7(9):1321-5. doi: 10.4161/cbt.7.9.6954. Epub 2008 Sep 8. Cancer Biol Ther. 2008. PMID: 18836294 Review.
Cited by
- Understanding the role of tumor stem cells in glioblastoma multiforme: a review article.
Fatoo A, Nanaszko MJ, Allen BB, Mok CL, Bukanova EN, Beyene R, Moliterno JA, Boockvar JA. Fatoo A, et al. J Neurooncol. 2011 Jul;103(3):397-408. doi: 10.1007/s11060-010-0406-3. Epub 2010 Sep 18. J Neurooncol. 2011. PMID: 20853017 Review. - Increased radiosensitivity and impaired DNA repair in patients with STAT3-LOF and ZNF341 deficiency, potentially contributing to malignant transformations.
Cekic S, Huriyet H, Hortoglu M, Kasap N, Ozen A, Karakoc-Aydiner E, Metin A, Ocakoglu G, Demiroz Abakay C, Temel SG, Ozemri Sag S, Baris S, Cavas T, Sebnem Kilic S. Cekic S, et al. Clin Exp Immunol. 2022 Jul 22;209(1):83-89. doi: 10.1093/cei/uxac041. Clin Exp Immunol. 2022. PMID: 35511492 Free PMC article. - Interleukin-6 Induced Proliferation Is Attenuated by Transforming Growth Factor-β-Induced Signaling in Human Hepatocellular Carcinoma Cells.
Srivastava A, Sharma H, Khanna S, Sadhu Balasundaram T, Chowdhury S, Chowdhury R, Mukherjee S. Srivastava A, et al. Front Oncol. 2022 Jan 20;11:811941. doi: 10.3389/fonc.2021.811941. eCollection 2021. Front Oncol. 2022. PMID: 35127527 Free PMC article. - STAT3 tyrosine phosphorylation influences survival in glioblastoma.
Birner P, Toumangelova-Uzeir K, Natchev S, Guentchev M. Birner P, et al. J Neurooncol. 2010 Dec;100(3):339-43. doi: 10.1007/s11060-010-0195-8. Epub 2010 May 9. J Neurooncol. 2010. PMID: 20455003 - Nuclear trafficking of the epidermal growth factor receptor family membrane proteins.
Wang YN, Yamaguchi H, Hsu JM, Hung MC. Wang YN, et al. Oncogene. 2010 Jul 15;29(28):3997-4006. doi: 10.1038/onc.2010.157. Epub 2010 May 17. Oncogene. 2010. PMID: 20473332 Free PMC article. Review.
References
- Bachoo R.M., Maher E.A., Ligon K.L., Sharpless N.E., Chan S.S., You M.J., Tang Y., DeFrances J., Stover E., Weissleder R., et al. Epidermal growth factor receptor and Ink4a/Arf. Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. - PubMed
- Bajenaru M.L., Hernandez M.R., Perry A., Zhu Y., Parada L.F., Garbow J.R., Gutmann D.H. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. - PubMed
- Bonni A., Sun Y., Nadal-Vicens M., Bhatt A., Frank D.A., Rozovsky I., Stahl N., Yancopoulos G.D., Greenberg M.E. Regulation of gliogenesis in the central nervous system by the JAK–STAT signaling pathway. Science. 1997;278:477–483. - PubMed
- Bowman T., Garcia R., Turkson J., Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. - PubMed
- Bromberg J.F. Activation of STAT proteins and growth control. Bioessays. 2001;23:161–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007753/GM/NIGMS NIH HHS/United States
- NS051255/NS/NINDS NIH HHS/United States
- R01 NS047188/NS/NINDS NIH HHS/United States
- NS41021/NS/NINDS NIH HHS/United States
- NS047188/NS/NINDS NIH HHS/United States
- R01 NS041021/NS/NINDS NIH HHS/United States
- R01 NS051255/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous