Activation of innate immunity (NK/IFN-gamma) in rat allogeneic liver transplantation: contribution to liver injury and suppression of hepatocyte proliferation - PubMed (original) (raw)
Comparative Study
Activation of innate immunity (NK/IFN-gamma) in rat allogeneic liver transplantation: contribution to liver injury and suppression of hepatocyte proliferation
Kezhen Shen et al. Am J Physiol Gastrointest Liver Physiol. 2008 Apr.
Abstract
Liver transplantation is presently the only curative treatment for patients with end-stage liver disease. However, the mechanisms underlying liver injury and hepatocyte proliferation posttransplantation remain obscure. In this investigation, liver injury and hepatocyte proliferation in syngeneic and allogeneic animal models were compared. Male Lewis and Dark Agouti (DA) rats were subjected to orthotopic liver transplantation (OLT). Rat OLT was performed in syngeneic (Lewis-Lewis) and allogeneic (Lewis-DA or DA-Lewis) animal models. Allogeneic liver grafts exhibited greater injury and cellular apoptosis than syngeneic grafts but less hepatocyte proliferation after OLT. Expression of IFN-gamma mRNA and activation of the downstream signal transducer and activator of transcription 1 (STAT1) and genes (interferon regulatory factor-1 and cyclin-dependent kinase inhibitor p21(CDKN1A)) were also greater in the allogeneic grafts compared with the syngeneic grafts. In contrast, STAT3 activation was lower in the allogeneic grafts. Furthermore, in the allogeneic grafts, depletion of natural killer (NK) cells decreased IFN-gamma/STAT1 activation but enhanced hepatocyte proliferation. These findings suggest that, compared with syngeneic transplantation, innate immunity (NK/IFN-gamma) is activated after allogeneic transplantation, which likely contributes to liver injury and inhibits hepatocyte proliferation.
Figures
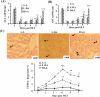
Figure 1
Liver injury and apoptosis are higher in the allogeneic grafts vs. the syngeneic grafts. Three pairs of OLT were performed (Lewis to Lewis: L-L; Lewis to DA: L-DA; DA to Lewis: DA-L) for various time points. (A, B) Sera were harvested for ALT/AST measurement. (C) Liver tissues were harvested and stained with TUNEL assay to examine apoptosis. Representative photomicrographs are shown in the top panel (arrows indicate TUNEL+ hepatocytes). The number of TUNEL+ hepatocytes was counted and shown in the bottom panel. Values in panels A-C are shown as means ± SEM (n=3 pairs on day 1; n=5 pairs on day 2 and 5; n=6 pairs on day 3, 4, and 7). *P<0.05, **P<0.01, ***P<0.001 compared with values from corresponding L-L syngeneic grafts.
Figure 2
Hepatocyte proliferation is lower in the allogeneic grafts vs. the syngeneic grafts. The liver tissues from Fig. 1 were stained with anti-PCNA or anti-Ki67 antibodies. Representative photomicrographs are shown in left panel. The numbers of Ki67+ and PCNA+ hepatocytes were counted and shown in right panel. Values are shown as means ± SEM (n=3−6 as described in Fig. 1). **P<0.01, ***P<0.001 compared with values from corresponding L-L syngeneic grafts.
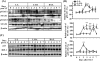
Figure 3
Upregulation of STAT1 activation and downregulation of STAT3 activation in the allogeneic grafts vs. the syngeneic grafts. The liver tissues from Fig. 1 were subject to Western blotting (A, B) and RT-PCR analyses (C). A representative of 3 experiments with similar results is shown. The band densities in panel A were quantified and the ratio of pSTAT1 (or STAT1, pSTAT3)/β actin mRNA were calculated. The ratios on day 0 were designated as 100%. Values are shown as means ± SEM (n=3−6 as described in Fig. 1). *P<0.05, **P<0.01 compared with values from corresponding L-L syngeneic grafts.
Figure 4
Upregulation of hepatic IFN-γ mRNA and serum IFN-γ levels in the allogeneic groups vs. the syngeneic groups. (A) The liver tissues from Fig. 1 were subject to RT-PCR analyses. A representative of 3 experiments with similar results is shown. (B) Sera from these transplanted rats were used for IFN-γ measurement. Values are shown as means ± SEM (n=3−6 as described in Fig. 1). *P<0.05, **P<0.01, ***P<0.001 compared with values from corresponding L-L syngeneic grafts.
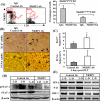
Figure 5
Depletion of NK cells enhances liver regeneration with deceasing STAT1 activation and IFN-γ/p21/IRF-1 mRNA expression in the allogeneic grafts. (A) Lewis and DA rats were treated with anti-NKR1P antibodies or IgG control antibodies. Twenty-four hours later, liver lymphocytes were isolated and analyzed with anti-CD3 and anti-NKRP1 antibodies. The representative flow cytometric results from DA rats are shown in the left panel. A summary of 3 independent experiments for the percentage of NK (NKRP1highCD3−) and NKT (NKRP1mediumCD3+) cells is shown in the right panel. (B-E) The livers from anti-NKR1P antibody- or control IgG-treated Lewis rats were transplanted into anti-NKRP1- or IgG-treated DA rats. Three days later, the transplanted DA rats were killed and livers harvested for immunostaining with anti-Ki67 or anti-PCNA antibodies. Representative photomicrographs are shown in panel B (arrows indicate Ki67+ or PCNA+ hepatocytes). The numbers of Ki67+ and PCNA+ hepatocytes were counted and are shown in C. Values are shown as means ± SEM (n=3−4). *P<0.05 compared with the corresponding IgG-treated allografts. Liver tissues were also subject to Western blotting (D) or RT-PCR analyses (E). A representative of 3 experiments with similar results is shown.
Similar articles
- Regulation of hepatocyte fate by interferon-γ.
Horras CJ, Lamb CL, Mitchell KA. Horras CJ, et al. Cytokine Growth Factor Rev. 2011 Feb;22(1):35-43. doi: 10.1016/j.cytogfr.2011.01.001. Epub 2011 Feb 18. Cytokine Growth Factor Rev. 2011. PMID: 21334249 Free PMC article. Review. - IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses.
Obara H, Nagasaki K, Hsieh CL, Ogura Y, Esquivel CO, Martinez OM, Krams SM. Obara H, et al. Am J Transplant. 2005 Sep;5(9):2094-103. doi: 10.1111/j.1600-6143.2005.00995.x. Am J Transplant. 2005. PMID: 16095488 Free PMC article. - Zinc finger protein A20 promotes regeneration of small-for-size liver allograft and suppresses rejection and results in a longer survival in recipient rats.
Xu MQ, Yan LN, Gou XH, Li DH, Huang YC, Hu HY, Wang LY, Han L. Xu MQ, et al. J Surg Res. 2009 Mar;152(1):35-45. doi: 10.1016/j.jss.2008.04.029. Epub 2008 Jun 10. J Surg Res. 2009. PMID: 19027921 - Peak protein expression of IL-2 and IFN-gamma correlate with the peak rejection episode in a spontaneously tolerant model of rat liver transplantation.
Lord R, Goto S, Pan T, Chiang K, Chen C, Sunagawa M. Lord R, et al. Cytokine. 2001 Feb 7;13(3):155-61. doi: 10.1006/cyto.2000.0815. Cytokine. 2001. PMID: 11161458 - Augmented regeneration of partial liver allograft induced by nuclear factor-kappaB decoy oligodeoxynucleotides-modified dendritic cells.
Xu MQ, Suo YP, Gong JP, Zhang MM, Yan LN. Xu MQ, et al. World J Gastroenterol. 2004 Feb 15;10(4):573-8. doi: 10.3748/wjg.v10.i4.573. World J Gastroenterol. 2004. PMID: 14966919 Free PMC article.
Cited by
- Dynamic immune cell profiling identified natural killer cell shift as the key event in early allograft dysfunction after liver transplantation.
Lu D, Yang X, Pan L, Lian Z, Tan W, Zhuo J, Yang M, Lin Z, Wei Q, Chen J, Zheng S, Xu X. Lu D, et al. Cell Prolif. 2024 Apr;57(4):e13568. doi: 10.1111/cpr.13568. Epub 2023 Oct 31. Cell Prolif. 2024. PMID: 37905596 Free PMC article. - Regulation of hepatocyte fate by interferon-γ.
Horras CJ, Lamb CL, Mitchell KA. Horras CJ, et al. Cytokine Growth Factor Rev. 2011 Feb;22(1):35-43. doi: 10.1016/j.cytogfr.2011.01.001. Epub 2011 Feb 18. Cytokine Growth Factor Rev. 2011. PMID: 21334249 Free PMC article. Review. - Liver mesenchymal stem cells are superior inhibitors of NK cell functions through differences in their secretome compared to other mesenchymal stem cells.
Yigitbilek F, Ozdogan E, Abrol N, Park WD, Hansen MJ, Dasari S, Stegall MD, Taner T. Yigitbilek F, et al. Front Immunol. 2022 Sep 21;13:952262. doi: 10.3389/fimmu.2022.952262. eCollection 2022. Front Immunol. 2022. PMID: 36211345 Free PMC article. - Protective effect of remote limb ischemic perconditioning on the liver grafts of rats with a novel model.
Jia J, Li J, Jiang L, Zhang J, Chen S, Wang L, Zhou Y, Xie H, Zhou L, Zheng S. Jia J, et al. PLoS One. 2015 Mar 18;10(3):e0121972. doi: 10.1371/journal.pone.0121972. eCollection 2015. PLoS One. 2015. PMID: 25785455 Free PMC article. - Activation of IFN-γ/STAT/IRF-1 in hepatic responses to Klebsiella pneumoniae infection.
Lin YC, Lu MC, Lin C, Chiang MK, Jan MS, Tang HL, Liu HC, Lin WL, Huang CY, Chen CM, Lai YC. Lin YC, et al. PLoS One. 2013 Nov 6;8(11):e79961. doi: 10.1371/journal.pone.0079961. eCollection 2013. PLoS One. 2013. PMID: 24223208 Free PMC article.
References
- Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:194–213. - PubMed
- Bishop GA, Wang C, Sharland AF, McCaughan G. Spontaneous acceptance of liver transplants in rodents: evidence that liver leucocytes induce recipient T-cell death by neglect. Immunol Cell Biol. 2002;80:93–100. - PubMed
- Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–715. - PubMed
- Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. - PubMed
- Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous