AR, the cell cycle, and prostate cancer - PubMed (original) (raw)
Review
AR, the cell cycle, and prostate cancer
Steven P Balk et al. Nucl Recept Signal. 2008.
Abstract
The androgen receptor (AR) is a critical effector of prostate cancer development and progression. The dependence of this tumor type on AR activity is exploited in treatment of disseminated prostate cancers, wherein ablation of AR function (achieved either through ligand depletion and/or the use of AR antagonists) is the first line of therapeutic intervention. These strategies are initially effective, and induce a mixed response of cell cycle arrest or apoptosis in prostate cancer cells. However, recurrent, incurable tumors ultimately arise as a result of inappropriately restored AR function. Based on these observations, it is imperative to define the mechanisms by which AR controls cancer cell proliferation. Mechanistic investigation has revealed that AR acts as a master regulator of G1-S phase progression, able to induce signals that promote G1 cyclin-dependent kinase (CDK) activity, induce phosphorylation/inactivation of the retinoblastoma tumor suppressor (RB), and thereby govern androgen-dependent proliferation. These functions appear to be independent of the recently identified TMPRSS2-ETS fusions. Once engaged, several components of the cell cycle machinery actively modulate AR activity throughout the cell cycle, thus indicating that crosstalk between the AR and cell cycle pathways likely modulate the mitogenic response to androgen. As will be discussed, discrete aberrations in this process can alter the proliferative response to androgen, and potentially subvert hormonal control of tumor progression.
Figures
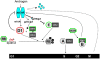
Figure 1. AR-cell cycle crosstalk.
Activated AR stimulates the accumulation of cyclin D1 (D1), through mammalian Target of Rapamycin (mTOR), to activate CDK4 and promote phosphorylation of the retinoblastoma (RB) tumor suppressor. In addition, AR-induced expression of p21Cip1 and degradation of p27Kip1 further enhance cycD1/CDK4 and cycE/CDK2-dependent inactivation of RB and allow expression of E2F target genes like cyclin A (CycA). Cyclin A in turn activates CDK2 to drive G1-S phase transition. Subsequently engaged components of the cell cycle machinery then impinge on AR to regulate the androgen response. Elevated cyclin D1 acts as in a negative feedback loop to attenuate AR activity, thereby modulating androgen action. In G2-phase, CDK1 promotes the phosphorylation and activation of AR. However, AR is degraded in M-phase and is purposed to be a “licensing factor” for DNA replication. Components that suppress AR function are outlined in red, whereas positive effectors of AR activity are outlined in green.
Similar articles
- Differential requirements for ras and the retinoblastoma tumor suppressor protein in the androgen dependence of prostatic adenocarcinoma cells.
Fribourg AF, Knudsen KE, Strobeck MW, Lindhorst CM, Knudsen ES. Fribourg AF, et al. Cell Growth Differ. 2000 Jul;11(7):361-72. Cell Growth Differ. 2000. PMID: 10939590 - The complex role of AR signaling after cytotoxic insult: implications for cell-cycle-based chemotherapeutics.
Comstock CE, Knudsen KE. Comstock CE, et al. Cell Cycle. 2007 Jun 1;6(11):1307-13. doi: 10.4161/cc.6.11.4353. Epub 2007 Jun 26. Cell Cycle. 2007. PMID: 17568191 Review. - Annexin-A7 protects normal prostate cells and induces distinct patterns of RB-associated cytotoxicity in androgen-sensitive and -resistant prostate cancer cells.
Torosyan Y, Simakova O, Naga S, Mezhevaya K, Leighton X, Diaz J, Huang W, Pollard H, Srivastava M. Torosyan Y, et al. Int J Cancer. 2009 Dec 1;125(11):2528-39. doi: 10.1002/ijc.24592. Int J Cancer. 2009. PMID: 19610065 - Cyclin D3 action in androgen receptor regulation and prostate cancer.
Olshavsky NA, Groh EM, Comstock CE, Morey LM, Wang Y, Revelo MP, Burd C, Meller J, Knudsen KE. Olshavsky NA, et al. Oncogene. 2008 May 15;27(22):3111-21. doi: 10.1038/sj.onc.1210981. Epub 2007 Dec 17. Oncogene. 2008. PMID: 18084330 - The role of tumor suppressor dysregulation in prostate cancer progression.
Dean JL, Knudsen KE. Dean JL, et al. Curr Drug Targets. 2013 Apr;14(4):460-71. doi: 10.2174/1389450111314040007. Curr Drug Targets. 2013. PMID: 23410128 Review.
Cited by
- ARe we there yet? Understanding androgen receptor signaling in breast cancer.
Michmerhuizen AR, Spratt DE, Pierce LJ, Speers CW. Michmerhuizen AR, et al. NPJ Breast Cancer. 2020 Sep 25;6:47. doi: 10.1038/s41523-020-00190-9. eCollection 2020. NPJ Breast Cancer. 2020. PMID: 33062889 Free PMC article. Review. - Simultaneous targeting of androgen receptor (AR) and MAPK-interacting kinases (MNKs) by novel retinamides inhibits growth of human prostate cancer cell lines.
Ramamurthy VP, Ramalingam S, Gediya L, Kwegyir-Afful AK, Njar VC. Ramamurthy VP, et al. Oncotarget. 2015 Feb 20;6(5):3195-210. doi: 10.18632/oncotarget.3084. Oncotarget. 2015. PMID: 25605250 Free PMC article. - Carcinogenesis in prostate cancer: The role of long non-coding RNAs.
Aird J, Baird AM, Lim MCJ, McDermott R, Finn SP, Gray SG. Aird J, et al. Noncoding RNA Res. 2018 Feb 1;3(1):29-38. doi: 10.1016/j.ncrna.2018.01.001. eCollection 2018 Mar. Noncoding RNA Res. 2018. PMID: 30159437 Free PMC article. Review. - Pathogenesis of prostate cancer: lessons from basic research.
Velcheti V, Karnik S, Bardot SF, Prakash O. Velcheti V, et al. Ochsner J. 2008 Winter;8(4):213-8. Ochsner J. 2008. PMID: 21603505 Free PMC article. - SRF inhibitors reduce prostate cancer cell proliferation through cell cycle arrest in an isogenic model of castrate-resistant prostate cancer.
Azam H, Maher S, Clarke S, Gallagher WM, Prencipe M. Azam H, et al. Cell Cycle. 2023 Jul-Aug;22(14-16):1759-1776. doi: 10.1080/15384101.2023.2229713. Epub 2023 Jun 28. Cell Cycle. 2023. PMID: 37377210 Free PMC article.
References
- Aaltomaa S., Lipponen P., Eskelinen M., Ala-Opas M., Kosma V. M. Prognostic value and expression of p21(waf1/cip1) protein in prostate cancer. Prostate. 1999;39:8–15. - PubMed
- Agus D. B., Cordon-Cardo C., Fox W., Drobnjak M., Koff A., Golde D. W., Scher H. I. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;91:1869–76. - PubMed
- Alt J. R., Gladden A. B., Diehl J. A. p21(Cip1) Promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J Biol Chem. 2002;277:8517–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials