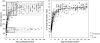Hominin life history: reconstruction and evolution - PubMed (original) (raw)
Review
Hominin life history: reconstruction and evolution
Shannen L Robson et al. J Anat. 2008 Apr.
Abstract
In this review we attempt to reconstruct the evolutionary history of hominin life history from extant and fossil evidence. We utilize demographic life history theory and distinguish life history variables, traits such as weaning, age at sexual maturity, and life span, from life history-related variables such as body mass, brain growth, and dental development. The latter are either linked with, or can be used to make inferences about, life history, thus providing an opportunity for estimating life history parameters in fossil taxa. We compare the life history variables of modern great apes and identify traits that are likely to be shared by the last common ancestor of Pan-Homo and those likely to be derived in hominins. All great apes exhibit slow life histories and we infer this to be true of the last common ancestor of Pan-Homo and the stem hominin. Modern human life histories are even slower, exhibiting distinctively long post-menopausal life spans and later ages at maturity, pointing to a reduction in adult mortality since the Pan-Homo split. We suggest that lower adult mortality, distinctively short interbirth intervals, and early weaning characteristic of modern humans are derived features resulting from cooperative breeding. We evaluate the fidelity of three life history-related variables, body mass, brain growth and dental development, with the life history parameters of living great apes. We found that body mass is the best predictor of great ape life history events. Brain growth trajectories and dental development and eruption are weakly related proxies and inferences from them should be made with caution. We evaluate the evidence of life history-related variables available for extinct species and find that prior to the transitional hominins there is no evidence of any hominin taxon possessing a body size, brain size or aspects of dental development much different from what we assume to be the primitive life history pattern for the Pan-Homo clade. Data for life history-related variables among the transitional hominin grade are consistent and none agrees with a modern human pattern. Aside from mean body mass, adult brain size, crown and root formation times, and the timing and sequence of dental eruption of Homo erectus are inconsistent with that of modern humans. Homo antecessor fossil material suggests a brain size similar to that of Homo erectus s. s., and crown formation times that are not yet modern, though there is some evidence of modern human-like timing of tooth formation and eruption. The body sizes, brain sizes, and dental development of Homo heidelbergensis and Homo neanderthalensis are consistent with a modern human life history but samples are too small to be certain that they have life histories within the modern human range. As more life history-related variable information for hominin species accumulates we are discovering that they can also have distinctive life histories that do not conform to any living model. At least one extinct hominin subclade, Paranthropus, has a pattern of dental life history-related variables that most likely set it apart from the life histories of both modern humans and chimpanzees.
Figures
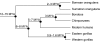
Fig. 1
Phylogenetic relationships of the extant great ape species. Estimated time of divergence of the hominid lineage from Glazko & Nei (2003), for chimpanzee/bonobo from Wildman et al. (2003), for the Bornean/Sumatran orangutans from Zhang et al. (2001), and for the eastern/western gorillas from Thalmann et al. (2007).
Fig. 2
Comparison of modern human and chimpanzee absolute (panel A) and relative (panel B) brain growth trajectories. Black triangles are chimpanzees (Herndon et al. 1999; n = 26; males = 16, females = 10); open circles are modern humans (Marchand, 1902; n = 160; males = 111, females = 49). Shaded bands in panel A represent the period of subadulthood with the duration of brain growth outlined and darkened.
Fig. 3
The more speciose (splitting) taxonomy. Informal groupings are based on brain size, body mass, postcanine tooth-size estimates, and locomotor mode. No ancestor-descendant relationships are implied among taxa.
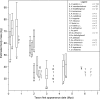
Fig. 4
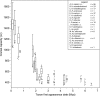
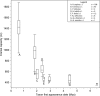
Estimated body mass plotted against first appearance date for the fossil hominin taxa recognized in the splitting taxonomy. Box and whisker plots show the median, upper, and lower quartiles (box) and the maximum and minimum values (whiskers). The number of individual estimates (n) used for each variable in this comparison is listed in the legend. Taxa represented by a single horizontal line have only a single estimate for this variable. Taxa with no data for this variable appear between question marks; their position along the vertical axis is determined by their informal group membership.
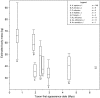
Fig. 5
Estimated body mass plotted against first appearance date for the fossil hominin taxa recognized in the lumping taxonomy. Box and whisker plots show the median, upper, and lower quartiles (box) and the maximum and minimum values (whiskers). The number of individual estimates (n) used for each variable in this comparison is listed in the legend. Taxa represented by a single horizontal line have only a single estimate for this variable. Taxa with no data for this variable appear between question marks; their position along the vertical axis is determined by their informal group membership.
Fig. 6
Estimated endocranial volume plotted against first appearance date for the fossil hominin taxa recognized in the splitting taxonomy. Box and whisker plots show the median, upper, and lower quartiles (box) and the maximum and minimum values (whiskers). The number of individual estimates (n) used for each variable in this comparison is listed in the legend. Taxa represented by a single horizontal line have only a single estimate for this variable. Taxa with no data for this variable appear between question marks; their position along the vertical axis is determined by their informal group membership.
Fig. 7
Estimated endocranial volume plotted against first appearance date for the fossil hominin taxa recognized in the lumping taxonomy. Box and whisker plots show the median, upper, and lower quartiles (box) and the maximum and minimum values (whiskers). The number of individual estimates (n) used for each variable in this comparison is listed in the legend. Taxa represented by a single horizontal line have only a single estimate for this variable.
Fig. 8
The relationship between crown formation and eruption sequence in modern humans, Pan, and P. boisei. The vertical dashed line represents the time from the onset of crown formation to eruption. The height of the crown represents the approximate time taken for crown formation; the balance of the period to eruption represents the time taken for the root to form. The tooth crowns are approximately to scale. Infancy is taken to cease at the time of M1 eruption (*). The vertical gray bars indicate rates and patterns in common among the taxa. All three genera share similar molar crown formation times, but Pan differs from the other two in eruption schedules and Homo in root formation times. Adapted from Macho & Wood (1995b).
Similar articles
- Inferences regarding the diet of extinct hominins: structural and functional trends in dental and mandibular morphology within the hominin clade.
Lucas PW, Constantino PJ, Wood BA. Lucas PW, et al. J Anat. 2008 Apr;212(4):486-500. doi: 10.1111/j.1469-7580.2008.00877.x. J Anat. 2008. PMID: 18380867 Free PMC article. Review. - The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo.
Tocheri MW, Orr CM, Jacofsky MC, Marzke MW. Tocheri MW, et al. J Anat. 2008 Apr;212(4):544-62. doi: 10.1111/j.1469-7580.2008.00865.x. J Anat. 2008. PMID: 18380869 Free PMC article. Review. - Dental development in Homo naledi.
Cofran Z, Walker CS. Cofran Z, et al. Biol Lett. 2017 Aug;13(8):20170339. doi: 10.1098/rsbl.2017.0339. Epub 2017 Aug 30. Biol Lett. 2017. PMID: 28855415 Free PMC article. - Evolution of M1 crown size and cusp proportions in the genus Homo.
Quam R, Bailey S, Wood B. Quam R, et al. J Anat. 2009 May;214(5):655-70. doi: 10.1111/j.1469-7580.2009.01064.x. J Anat. 2009. PMID: 19438761 Free PMC article. - A proper study for mankind: Analogies from the Papionin monkeys and their implications for human evolution.
Jolly CJ. Jolly CJ. Am J Phys Anthropol. 2001;Suppl 33:177-204. doi: 10.1002/ajpa.10021. Am J Phys Anthropol. 2001. PMID: 11786995 Review.
Cited by
- Dental evidence for ontogenetic differences between modern humans and Neanderthals.
Smith TM, Tafforeau P, Reid DJ, Pouech J, Lazzari V, Zermeno JP, Guatelli-Steinberg D, Olejniczak AJ, Hoffman A, Radovcic J, Makaremi M, Toussaint M, Stringer C, Hublin JJ. Smith TM, et al. Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):20923-8. doi: 10.1073/pnas.1010906107. Epub 2010 Nov 15. Proc Natl Acad Sci U S A. 2010. PMID: 21078988 Free PMC article. - Growth and development of the third permanent molar in Paranthropus robustus from Swartkrans, South Africa.
Dean C, Zanolli C, Le Cabec A, Tawane M, Garrevoet J, Mazurier A, Macchiarelli R. Dean C, et al. Sci Rep. 2020 Nov 4;10(1):19053. doi: 10.1038/s41598-020-76032-2. Sci Rep. 2020. PMID: 33149180 Free PMC article. - The Life History of Learning Subsistence Skills among Hadza and BaYaka Foragers from Tanzania and the Republic of Congo.
Lew-Levy S, Ringen EJ, Crittenden AN, Mabulla IA, Broesch T, Kline MA. Lew-Levy S, et al. Hum Nat. 2021 Mar;32(1):16-47. doi: 10.1007/s12110-021-09386-9. Epub 2021 May 13. Hum Nat. 2021. PMID: 33982236 Free PMC article. - Colloquium paper: how grandmother effects plus individual variation in frailty shape fertility and mortality: guidance from human-chimpanzee comparisons.
Hawkes K. Hawkes K. Proc Natl Acad Sci U S A. 2010 May 11;107 Suppl 2(Suppl 2):8977-84. doi: 10.1073/pnas.0914627107. Epub 2010 May 5. Proc Natl Acad Sci U S A. 2010. PMID: 20445089 Free PMC article. - Heterochrony in chimpanzee and bonobo spatial memory development.
Rosati AG. Rosati AG. Am J Phys Anthropol. 2019 Jun;169(2):302-321. doi: 10.1002/ajpa.23833. Epub 2019 Apr 11. Am J Phys Anthropol. 2019. PMID: 30973969 Free PMC article.
References
- Aiello LC, Dean C. An Introduction to Human Evolutionary Anatomy. London: Academic Press; 1990.
- Aiello L, Wood B. Cranial variables as predictors of hominine body mass. Am J Phys Anthropol. 1994;95:409–426. - PubMed
- Alemseged Z, Spoor F, Kimbel WH, et al. A juvenile early hominin skeleton from Dikika, Ethiopia. Nature. 2006;443:296–301. - PubMed
- Alvarez HP. Grandmother hypothesis and primate life histories. Am J Phys Anthropol. 2000;133:435–450. - PubMed
- Anemone RL. Dental development and life history in hominid evolution. In: McNamara KJ, editor. Human Evolution through Developmental Change. Baltimore: Johns Hopkins University Press; 2002. pp. 249–280.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous