Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis - PubMed (original) (raw)
Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis
Shari E Gelber et al. J Bacteriol. 2008 Jun.
Abstract
Pore-forming toxins are essential to the virulence of a wide variety of pathogenic bacteria. Gardnerella vaginalis is a bacterial species associated with bacterial vaginosis (BV) and its significant adverse sequelae, including preterm birth and acquisition of human immunodeficiency virus. G. vaginalis makes a protein toxin that generates host immune responses and has been hypothesized to be involved in the pathogenesis of BV. We demonstrate that G. vaginalis produces a toxin (vaginolysin [VLY]) that is a member of the cholesterol-dependent cytolysin (CDC) family, most closely related to intermedilysin from Streptococcus intermedius. Consistent with this predicted relationship, VLY lyses target cells in a species-specific manner, dependent upon the complement regulatory molecule CD59. In addition to causing erythrocyte lysis, VLY activates the conserved epithelial p38 mitogen-activated protein kinase pathway and induces interleukin-8 production by human epithelial cells. Transfection of human CD59 into nonsusceptible cells renders them sensitive to VLY-mediated lysis. In addition, a single amino acid substitution in the VLY undecapeptide [VLY(P480W)] generates a toxoid that does not form pores, and introduction of the analogous proline residue into another CDC, pneumolysin, significantly decreases its cytolytic activity. Further investigation of the mechanism of action of VLY may improve understanding of the functions of the CDC family as well as diagnosis and therapy for BV.
Figures
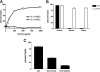
FIG. 1.
Phylogenetic relationship between VLY and other members of the CDC family. (A) Phylogram of full-length CDC protein sequences predicted by the neighbor joining algorithm. Numbers represent calculated relative phylogenetic distances. Abbreviations for CDC proteins: LLO, listeriolysin O; IVN, ivanolysin; SLG, seeligeriolysin; SPH, sphaericolysin; ALO, anthrolysin O; CER, cereolysin; PFO, perfringolysin O; ALV, alveolysin; TET, tetanolysin O; PYO, pyolysin; MLY, mitilysin; SLO, streptolysin O; SUI, suilysin; THU, thuringensolysin. (B) Multiple alignment of undecapeptide regions from known CDCs. The predicted amino acid sequence of VLY contains a variant undecapeptide region most similar to the undecapeptide from ILY. The sequence labeled “consensus” corresponds to the undecapeptide from MLY, PLY, SUI, IVN, ALV, SPH, THU, SLO, ALO, LLO, PFO, CER, and TET. (C) Western blot of lysed G. vaginalis bacteria (GV) and purified, recombinant VLY, probed with anti-PLY monoclonal antibody.
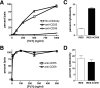
FIG. 2.
Human-specific, cholesterol-dependent hemolytic activity of VLY. (A) Washed human (hRBC) or sheep (sRBC) erythrocytes (1% solution in PBS) were exposed to the indicated concentrations of purified recombinant VLY for 30 min, followed by pelleting of cells. Hemoglobin release was measured as optical density at 415 nm of the supernatant and normalized to 100% lysis for each species tested (P < 0.01, ANOVA). (B) Erythrocytes from various species were exposed to VLY or the non-species-specific toxin PLY (both toxins at 5 μg/ml), and lysis was measured. (C) Addition of cholesterol (Ch) at 1 μg/ml or 10 μg/ml inhibits human erythrocyte lysis by VLY (5 μg/ml) (P < 0.001, ANOVA).
FIG. 3.
Host specificity of VLY depends on the complement regulatory molecule CD59. (A) VLY-induced lysis of human erythrocytes was inhibited by monoclonal antibody to human CD59 (P < 0.0001) but not antibody to another glycosylphosphatidylinositol-anchored cell surface antigen (CD55) or mock treatment (PBS). (B) Antibody to CD59 does not inhibit PLY-mediated lysis of human erythrocytes. (C) Lactate dehydrogenase release from Chinese hamster ovary (CHO) cells transfected with empty vector (IRES) or human CD59 (IRES-hCD59) and exposed to VLY (10 μg/ml) for 30 min. Transfection of human CD59 increases VLY-mediated lysis (_P_ < 0.0001). (D) Transfection of human CD59 into CHO cells does not affect PLY (1 μg/ml)-mediated lysis (_P_ > 0.05).
FIG. 4.
VLY-mediated epithelial cell activation and erythrocyte lysis require P480. (A) Human cervical epithelial cell line HeLa was treated for 30 min with medium alone (−), VLY, or VLY(P480W) (1 to 10 μg/ml) prior to lysis and Western blotting with antibodies specific for total (p38) and phospho-p38 (pp38) MAPK. (B) HeLa cells were treated with VLY or VLY(P480W) (10 μg/ml) for 2 h prior to RNA extraction and assay of relative quantity of IL-8 message by real-time PCR. (C) Human (hRBC) and sheep (sRBC) erythrocytes were treated with the indicated concentrations of VLY or VLY(P480W), and hemolysis was assessed as described above. (D) Human (hRBC) erythrocytes were treated with the indicated concentrations of PLY or PLY(W435P), and hemolysis was assessed as described above.
Similar articles
- Vaginolysin drives epithelial ultrastructural responses to Gardnerella vaginalis.
Randis TM, Zaklama J, LaRocca TJ, Los FC, Lewis EL, Desai P, Rampersaud R, Amaral FE, Ratner AJ. Randis TM, et al. Infect Immun. 2013 Dec;81(12):4544-50. doi: 10.1128/IAI.00627-13. Epub 2013 Sep 30. Infect Immun. 2013. PMID: 24082080 Free PMC article. - Antibody-based detection and inhibition of vaginolysin, the Gardnerella vaginalis cytolysin.
Randis TM, Kulkarni R, Aguilar JL, Ratner AJ. Randis TM, et al. PLoS One. 2009;4(4):e5207. doi: 10.1371/journal.pone.0005207. Epub 2009 Apr 16. PLoS One. 2009. PMID: 19370149 Free PMC article. - Interaction of Gardnerella vaginalis and Vaginolysin with the Apical versus Basolateral Face of a Three-Dimensional Model of Vaginal Epithelium.
Garcia EM, Kraskauskiene V, Koblinski JE, Jefferson KK. Garcia EM, et al. Infect Immun. 2019 Mar 25;87(4):e00646-18. doi: 10.1128/IAI.00646-18. Print 2019 Apr. Infect Immun. 2019. PMID: 30692180 Free PMC article. - Cholesterol-Dependent Cytolysins Produced by Vaginal Bacteria: Certainties and Controversies.
Pleckaityte M. Pleckaityte M. Front Cell Infect Microbiol. 2020 Jan 10;9:452. doi: 10.3389/fcimb.2019.00452. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 31998661 Free PMC article. Review. - Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes.
Kaneko J, Kamio Y. Kaneko J, et al. Biosci Biotechnol Biochem. 2004 May;68(5):981-1003. doi: 10.1271/bbb.68.981. Biosci Biotechnol Biochem. 2004. PMID: 15170101 Review.
Cited by
- Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates.
Castro J, Alves P, Sousa C, Cereija T, França Â, Jefferson KK, Cerca N. Castro J, et al. Sci Rep. 2015 Jun 26;5:11640. doi: 10.1038/srep11640. Sci Rep. 2015. PMID: 26113465 Free PMC article. - Antibiotic resistance and pathogenicity assessment of various Gardnerella sp. strains in local China.
Zhang K, Lu M, Zhu X, Wang K, Jie X, Li T, Dong H, Li R, Zhang F, Gu L. Zhang K, et al. Front Microbiol. 2022 Sep 26;13:1009798. doi: 10.3389/fmicb.2022.1009798. eCollection 2022. Front Microbiol. 2022. PMID: 36225381 Free PMC article. - More than a pore: the cellular response to cholesterol-dependent cytolysins.
Cassidy SK, O'Riordan MX. Cassidy SK, et al. Toxins (Basel). 2013 Apr 12;5(4):618-36. doi: 10.3390/toxins5040618. Toxins (Basel). 2013. PMID: 23584137 Free PMC article. Review. - The Female Vaginal Microbiome in Health and Bacterial Vaginosis.
Chen X, Lu Y, Chen T, Li R. Chen X, et al. Front Cell Infect Microbiol. 2021 Apr 7;11:631972. doi: 10.3389/fcimb.2021.631972. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33898328 Free PMC article. Review. - Changes of the vaginal microbiota in HPV infection and cervical intraepithelial neoplasia: a cross-sectional analysis.
Lin W, Zhang Q, Chen Y, Dong B, Xue H, Lei H, Lu Y, Wei X, Sun P. Lin W, et al. Sci Rep. 2022 Feb 18;12(1):2812. doi: 10.1038/s41598-022-06731-5. Sci Rep. 2022. PMID: 35181685 Free PMC article.
References
- Aroutcheva, A. A., J. A. Simoes, K. Behbakht, and S. Faro. 2001. Gardnerella vaginalis isolated from patients with bacterial vaginosis and from patients with healthy vaginal ecosystems. Clin. Infect. Dis. 331022-1027. - PubMed
- Billington, S. J., J. G. Songer, and B. H. Jost. 2002. The variant undecapeptide sequence of the Arcanobacterium pyogenes haemolysin, pyolysin, is required for full cytolytic activity. Microbiology 1483947-3954. - PubMed
- Bradshaw, C. S., A. N. Morton, J. Hocking, S. M. Garland, M. B. Morris, L. M. Moss, L. B. Horvath, I. Kuzevska, and C. K. Fairley. 2006. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 1931478-1486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous