Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes - PubMed (original) (raw)
Review
Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes
Adilson Guilherme et al. Nat Rev Mol Cell Biol. 2008 May.
Abstract
Acquired resistance to the action of insulin to stimulate glucose transport in skeletal muscle is associated with obesity and promotes the development of type 2 diabetes. In skeletal muscle, insulin resistance can result from high levels of circulating fatty acids that disrupt insulin signalling pathways. However, the severity of insulin resistance varies greatly among obese people. Here we postulate that this variability might reflect differences in levels of lipid-droplet proteins that promote the sequestration of fatty acids within adipocytes in the form of triglycerides, thereby lowering exposure of skeletal muscle to the inhibitory effects of fatty acids.
Figures
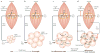
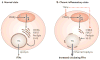
Figure 1. Chronic inflammation in adipose tissue triggers insulin resistance in skeletal muscle
a | In the lean state, small adipocytes efficiently store fatty acids as triglyceride (TG input, arrow), which can be mobilized and used to generate ATP through the mitochondrial β-oxidation pathway in muscle during periods of caloric need. Insulin-stimulated glucose uptake under these conditions is normal. b | Excess caloric intake leads to metabolic overload, increased TG input and adipocyte enlargement. Nonetheless, in non-diabetic overweight individuals, TG storage by adipose cells and β-oxidation in muscle can often be maintained to prevent insulin resistance. c | On further overloading with TG, hypertrophy of adipocytes and increased secretion of macrophage chemoattractants occurs, including the secretion of monocyte chemoattractant protein-1 (MCP-1; arrows), which recruits additional macrophages. d | Macrophage recruitment in turn results in a pro-inflammatory state in obese adipose tissue. Infiltrating macrophages secrete large amounts of tumour-necrosis factor-α (TNFα), which results in a chronic inflammatory state with impaired TG deposition and increased lipolysis (arrow and plus signal). The excess of circulating TG and free fatty acids results in the accumulation of activated lipids in the muscle (yellow dots), disrupting functions such as mitochondrial oxidative phosphorylation and insulin-stimulated glucose transport, thus triggering insulin resistance.
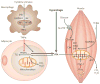
Figure 2. Chronic inflammation impairs triglyceride deposition in adipose tissue
The chronic inflammatory state induced by lipid overload in adipose cells triggers macrophage recruitment within the adipose tissue. Secreted cytokines stimulate macrophages to produce large amounts of tumour-necrosis factor-α (TNFα) through the IKKβ–NF-κB (inhibitor of nuclear factor (NF)-κB (IκB) kinase-β–NF-κB) and the JNK–AP1 (Jun N-terminal kinase–activator protein-1) signalling pathways. MAP4K4 (mitogen-activated protein kinase kinase kinase kinase-4) might also be required for TNFα production in activated macrophages. The macrophage-secreted TNFα enhances lipolysis and downregulates peroxisome proliferator-activated receptor-γ (PPARγ)-mediated triglyceride (TG) biosynthesis and storage in adipocytes. Hyperphagia, combined with enhanced lipolysis and impaired TG sequestration triggered by TNFα, results in increased levels of circulating free fatty acids (FFAs) and TG deposition in the muscle. Ectopic lipid and FFAs (yellow dots) attenuate expresssion of genes that are involved in mitochondrial function, such as PPARγ co-activator-1 (PGC-1); enhance ceramide (CM) biosynthesis and inhibit insulin-stimulated glucose transport through activation of the protein kinases protein kinase C (PKC), IKKβ and JNK. TNFα can also inhibit insulin-stimulated glucose transporter type-4 (GLUT4) glucose transport in muscle through activation of MAP4K4 and JNK kinases.
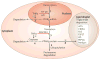
Figure 3. PPARγ downregulation by TNFα impairs triglyceride storage in adipose cells
Peroxisome proliferator-activated receptor-γ (_PPAR_γ) expression can be regulated at the transcriptional level by tumour-necrosis factor-α (TNFα) (arrow 1) through the activation of nuclear factor-κB (NF-κB) and activator protein-1 (AP1), which negatively regulate _PPAR_γ expression–. Recent data indicate rapid turnover of _PPAR_γ mRNA in adipocytes and treatment of cultured adipocytes with TNFα might enhance _PPAR_γ mRNA degradation (dashed arrow 2). Translational control of PPARγ that is mediated by MAP4K4 (mitogen-activated protein kinase kinase kinase kinase-4), a protein kinase that is upregulated by TNFα, can also occur (arrow 3). Furthermore, activation of caspases by TNFα signalling might trigger PPARγ protein degradation in adipocytes (dashed arrow 4). Regulation of PPARγ activity and stability are also negatively regulated by kinase-mediated phosphorylation and ubiquitylation, which promote PPARγ protein degradation through a proteasome-dependent pathway (arrow 5). TNFα action at multiple levels might therefore result in decreased PPARγ activity. Precise regulation of PPARγ expression and function can contribute to the control of triglyceride biosynthesis, hydrolysis and deposition in the lipid droplet — the lipid storage organelle of adipocytes. This can occur through the regulation of the expression of triglyceride metabolism enzymes such as phosphoenolpyruvate carboxykinase (PEPCK), fatty acid synthase (FAS), Acyl-CoA synthetase (ACS), lipoprotein lipase (LPL) and lipid-droplet proteins including CIDEA, FSP27 and perilipin (arrow 6). Dashed arrows that emanate from _PPAR_γ mRNA (step 2) and protein (step 4) indicate hypotheses that await definitive testing.
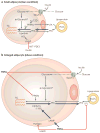
Figure 4. TNFα decreases triglyceride deposition and increases lipolysis in adipose cells
a | In small adipocytes (lean condition), insulin promotes free fatty acid (FFA) esterification into triglycerides (TG, schematically represented as the three-carbon glycerol backbone with three acyl fatty acid chains (wavy lines)) through stimulation of glucose transporter type-4 (GLUT4)-mediated glucose uptake. Glucose can be converted to α-glycerol phosphate, the main source of the glycerol backbone of TG. Peroxisome proliferator-activated receptor-γ (PPARγ) activates lipoprotein lipase (LPL) expression and the TG biosynthetic pathway. Secreted LPL hydrolyses TG from circulating very low-density lipoprotein (VLDL), releasing FFAs to be re-esterified. Several thiazolidinediones (TZDs) can activate PPARγ. Insulin signalling also downregulates TG lipolysis through hormone-sensitive lipase (HSL). Insulin stimulation of the phosphatidylinositol 3-kinase (PI3K)–AKT/protein kinase B (PKB) pathway leads to activation of the enzyme phosphodiesterase-3 (PDE3). This enzyme catalyses the breakdown of cyclic AMP (cAMP) which in turn reduces activation of HSL. b | In enlarged adipocytes from inflamed fat tissue, high levels of tumour necrosis factor-α (TNFα) result in decreased fatty acid esterification and enhanced lipolysis. GLUT4, LPL and PPARγ protein levels are attenuated by TNFα, resulting in decreased glucose transport and fatty acid esterification. TNFα also has a stimulatory effect on lipolysis by increasing the levels of cAMP and activation (plus signal) of HSL, combined with the downregulation of perilipin through activation of the mitogen-activated protein kinase (MAPK) pathway.
Figure 5. TNFα downregulates lipid-droplet proteins and enhances lipolysis in adipose cells
a | In the normal state, peroxisome proliferator-activated receptor-γ (PPARγ) function drives the expression of lipid-droplet proteins, such as FSP27, CIDEA, perilipin, ADRP and S3–12 in adipose cells. Their presence on lipid droplets inhibits basal lipolysis and free fatty acid (FFA) release, thereby promoting net triglyceride (TG) storage. b | We propose that in chronic inflammatory states, tumour-necrosis factor-α (TNFα) downregulates PPARγ function, attenuating the expression of these lipid-droplet proteins. With reduced levels of these proteins, their ability to shield and protect the lipid droplet from active lipases might contribute to enhanced TG lipolysis and circulating FFAs. Additionally, post-transcriptional regulation of lipid-droplet proteins by TNFα can occur (dashed inhibitory red line).
Similar articles
- [Adiponectin: from adipocyte to skeletal muscle].
Ferré P. Ferré P. Ann Endocrinol (Paris). 2004 Feb;65(1 Suppl):S36-43. doi: 10.1016/s0003-4266(04)95999-9. Ann Endocrinol (Paris). 2004. PMID: 15163922 Review. French. - Whey Peptides Stimulate Differentiation and Lipid Metabolism in Adipocytes and Ameliorate Lipotoxicity-Induced Insulin Resistance in Muscle Cells.
D'Souza K, Mercer A, Mawhinney H, Pulinilkunnil T, Udenigwe CC, Kienesberger PC. D'Souza K, et al. Nutrients. 2020 Feb 6;12(2):425. doi: 10.3390/nu12020425. Nutrients. 2020. PMID: 32041341 Free PMC article. - Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes.
Greenberg AS, McDaniel ML. Greenberg AS, et al. Eur J Clin Invest. 2002 Jun;32 Suppl 3:24-34. doi: 10.1046/j.1365-2362.32.s3.4.x. Eur J Clin Invest. 2002. PMID: 12028372 Review. - Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance.
Kelley DE, Goodpaster BH. Kelley DE, et al. Diabetes Care. 2001 May;24(5):933-41. doi: 10.2337/diacare.24.5.933. Diabetes Care. 2001. PMID: 11347757 Review.
Cited by
- Liver knockout of MCU leads to greater dysregulation of lipid metabolism in MAFLD.
Liao Q, Zhang Y, Pan T, Sun Y, Liu S, Zhang Z, Li Y, Yu L, Luo Z, Xiao Y, Qi X, Jiang T, Su S, Liu S, Qi X, Li X, Damba T, Batchuluun K, Liang Y, Wei S, Zhou L. Liao Q, et al. Sci Rep. 2024 Nov 15;14(1):28167. doi: 10.1038/s41598-024-78935-w. Sci Rep. 2024. PMID: 39548134 Free PMC article. - Myeloid-Derived Suppressor Cells (MDSCs) and Obesity-Induced Inflammation in Type 2 Diabetes.
Ghemiș L, Goriuc A, Minea B, Botnariu GE, Mârțu MA, Ențuc M, Cioloca D, Foia LG. Ghemiș L, et al. Diagnostics (Basel). 2024 Nov 1;14(21):2453. doi: 10.3390/diagnostics14212453. Diagnostics (Basel). 2024. PMID: 39518420 Free PMC article. Review. - Aerobic Exercise Prevents High-Fat-Diet-Induced Adipose Tissue Dysfunction in Male Mice.
Guo Q, Li N, Shi H, Gan Y, Wang W, Jia J, Zhou Y. Guo Q, et al. Nutrients. 2024 Oct 11;16(20):3451. doi: 10.3390/nu16203451. Nutrients. 2024. PMID: 39458447 Free PMC article. - Influence of Type 2 Diabetes and Adipose Tissue Dysfunction on Breast Cancer and Potential Benefits from Nutraceuticals Inducible in Microalgae.
Sergi D, Melloni M, Passaro A, Neri LM. Sergi D, et al. Nutrients. 2024 Sep 25;16(19):3243. doi: 10.3390/nu16193243. Nutrients. 2024. PMID: 39408212 Free PMC article. Review. - Evaluating the impact of chronic kidney disease and the triglyceride-glucose index on cardiovascular disease: mediation analysis in the NHANES.
Pei H, Su X, Wu S, Wang Z. Pei H, et al. BMC Public Health. 2024 Oct 9;24(1):2750. doi: 10.1186/s12889-024-20243-z. BMC Public Health. 2024. PMID: 39385084 Free PMC article.
References
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. - PubMed
- Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. - PubMed
- Alberti KG. The costs of non-insulin-dependent diabetes mellitus. Diabet Med. 1997;14:7–9. - PubMed
- Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocr Rev. 2007;28:48–83. - PubMed
- Sims EA, et al. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK030648-25/DK/NIDDK NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- R01 DK030648/DK/NIDDK NIH HHS/United States
- DK32520/DK/NIDDK NIH HHS/United States
- R37 DK030898-22/DK/NIDDK NIH HHS/United States
- R37 DK030898/DK/NIDDK NIH HHS/United States
- R01 DK030898/DK/NIDDK NIH HHS/United States
- P30 DK032520-25/DK/NIDDK NIH HHS/United States
- DK030648/DK/NIDDK NIH HHS/United States
- DK030898/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical