IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection - PubMed (original) (raw)
IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection
Kevin N Couper et al. PLoS Pathog. 2008.
Abstract
The outcome of malaria infection is determined, in part, by the balance of pro-inflammatory and regulatory immune responses. Failure to develop an effective pro-inflammatory response can lead to unrestricted parasite replication, whilst failure to regulate this response leads to the development of severe immunopathology. IL-10 and TGF-beta are known to be important components of the regulatory response, but the cellular source of these cytokines is still unknown. Here we have examined the role of natural and adaptive regulatory T cells in the control of malaria infection and find that classical CD4+CD25(hi) (and Foxp3+) regulatory T cells do not significantly influence the outcome of infections with the lethal (17XL) strain of Plasmodium yoelii (PyL). In contrast, we find that adaptive IL-10-producing, CD4+ T cells (which are CD25-, Foxp3-, and CD127- and do not produce Th1, Th2, or Th17 associated cytokines) that are generated during both PyL and non-lethal P. yoelii 17X (PyNL) infections are able to down-regulate pro-inflammatory responses and impede parasite clearance. In summary, we have identified a population of induced Foxp3- regulatory (Tr1) T cells, characterised by production of IL-10 and down regulation of IL-7Ralpha, that modulates the inflammatory response to malaria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
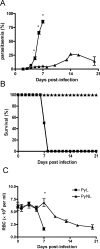
Figure 1. Course of infection of lethal (PyL) and non-lethal (PyNL) P. yoelii in C57BL/6 mice.
C57BL/6 mice were infected i.v. with 104 P. yoelii 17XL (PyL) or P. yoelii 17X (PyNL) parasites. The course of each infection was followed by monitoring (A) parasitaemia, (B) survival and(C) anaemia. 4–5 mice per group. Results are representative of 4 separate experiments. * = significant differences (p<0.05) between PyL and PyNL.
Figure 2. Expansion and activation of natural Foxp3+ regulatory T cell populations during PyL and PyNL infections.
Numbers and activation status of Foxp3+ regulatory T cells were determined on various days post-infection with PyL or PyNL in (A–D) WT mice or (E–F) Foxp3-GFP transgenic mice. (A) Representative dot plot of intracellular staining in CD4+ T cells. On selected days post-infection with PyL or PYNL, splenic CD4+ lymphocytes were analysed for (B) numbers of Foxp3+ cells,(C) intensity (MFI) of Foxp3 staining and (D) ratio of Foxp3+ to Foxp3− cells. (E) Representative dot plots showing GFP (Foxp3) expression in CD4+ splenocytes from 7 day PyL and PyNL-infected animals and uninfected controls. (F) Numbers of splenic GFP+ (Foxp3+) CD4+ regulatory T cells on day 5 post infection. 3–5 mice per group. Results are representative of 3 independent experiments. Symbols represent significant differences (p<0.05) between groups: # PyL vs PyNL; * PyL vs uninfected; ∼ PyNL vs uninfected.
Figure 3. Natural Treg do not significantly contribute to the virulence of PyL infection.
(A–C) Mice were given either a single dose of 0.75 mg 7D4 (IgM clone) or 0.25 mg 7D4 combined with 0.75 mg PC61 (IgG1 clone) on 3 days prior to infection with 104 PyL pRBC. The effect of anti-CD25 treatment on the course of PyL infection was examined in (A–C) C57BL/6 mice by following (A) parasitaemia, (B) anaemia and (C) weight loss. Groups consisted of 3–5 mice and data are representative of 2 independent experiments. Symbols represent significant differences (p<0.05) between groups: (A–D) # 7D4 vs 7D4/PC61; * 7D4 vs PBS; ∼ 7D4/PC61 vs PBS; (G–H) # 7D4 vs 7D4/PC61; * 7D4 vs 7D4×3; ∼ 7D4 vs PBS; + 7D4/PC61 vs 7D4×3; Φ 7D4/PC61 vs PBS; Δ 7D4×3 vs PBS. (D–F) Naive CD4+CD25− (non-Treg) and CD4+CD25hi (Treg) cells were purified by flow cytometric cell sorting and adoptively transferred alone or at a 10∶1 ratio (non Treg∶Treg) into RAG-1−/− mice prior to infection with PyL parasites. (D) shows the purity of sorted CD4+CD25− and CD4+CD25hi populations prior to adoptive transfer and the relative expression of Foxp3 within the purified populations. The affect of adoptive transfer on the course of infection was determined by monitoring (E) parasitaemia and (F) survival for the duration of the experiment. Groups consisted of 5 mice and the results are representative of 2 independent experiments. Symbols represent significant differences (p<0.05) between groups: # CD25− vs CD25+; * CD25− vs CD25−/ CD25+; ∼ CD25− vs control; + CD25+ vs CD25−/ CD25+; Φ CD25+ vs control; Δ CD25−/ CD25+ vs control.
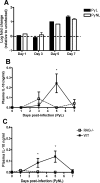
Figure 4. CD4+ T cells are a major source of IL-10 during both PyL and PyNL infection.
(A) On selected days post-infection, CD4+ T cells were purified from PyL and PyNL infected mice by MACS sorting and levels of IL-10 mRNA were determined by real time PCR (Taqman) relative to the house keeping gene GAPDH. The results are shown as the fold change in expression relative to uninfected naïve CD4+ T cells. In separate experiments the levels of IL-10 in the plasma of WT and RAG-1−/− mice were determined by ELISA on selected days of (B) PyL and (C) PyNL infection. Groups consisted of 3–5 mice and the results are representative of 2 separate experiments. For Taqman analysis, purified cells from several mice in each group were pooled; no significant differences between groups were identified. * indicates significant differences (p<0.05) between WT and RAG-1−/− infected mice.
Figure 5. CD127low Foxp3− CD4+ T cells that do not constitutively express CD25 are the major source of IL-10 during P. yoelii infection.
Transgenic, IL-10-GFP knockin tiger mice were infected with PyL or PyNL. (A) Splenic lymphocytes from infected or uninfected control mice were analysed for expression of CD4+ and GFP. (B,C) 7 days post infection, splenic CD4+ T cells were analysed for (B) expression of CD25 and Foxp3, or (C) GFP (IL-10) and CD25, CD69, CD62L or CD127. (D) The frequency and number of GFP+ (IL-10+) CD4+ T cells was calculated 7 days post infection in infected or uninfected mice. Groups consisted of 3–5 mice and the results are representative of 2 independent experiments.
Figure 6. IL-10 producing CD4+ T cells that develop during P. yoelii infection are Tr1 cells and not Th1, Th2, or Th17 cells.
Splenic CD4+ T cells were isolated from transgenic IL-10-GFP mice on day 7 of PyL or PyNL infection and were purified by flow cytometric cell sorting into GFP+ (IL-10+) and GFP− (IL-10−) populations. (A) shows the purity of the purified GFP+ and GFP− populations. (B) Expression of IL-10, Foxp3, IFN-γ, IL-4, IL-13 and IL-17A mRNA was determined by real time PCR (Taqman) relative to the house keeping gene, GAPDH. The results are shown as the fold change in expression relative to uninfected naïve CD4+ T cells. For Taqman analysis, purified cells from several mice in each group were pooled.
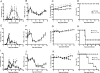
Figure 7. IL-10 impedes parasite clearance during both PyL and PyNL infection.
The course of infection with (A–D) PyNL and (E–L) PyL in mice given (A–H) 104 pRBC's or (I–L) 103 pRBC's was compared in WT and IL-10−/− mice by monitoring (A, E, I) parasitaemia (B, F, J) anaemia, (C, G, K) weight loss and ( D, H, L)survival. Groups consisted of 4–5 mice and the results are representative of 4 independent experiments. * indicates significant differences (p<0.05) between WT and IL-10−/− infected mice.
Figure 8. T cell-derived IL-10 inhibits parasite clearance during PyL and PyNL infections.
Prior to infection with (A–D) PyNL and (E–L) PyL with (A–H) 104 or (I–L) 103 pRBC's, RAG-1−/− mice received naïve CD4+ T cells that had been purified from either WT or IL-10−/− mice by magnetic bead sorting. The course of infection was followed by monitoring (A, E, I) parasitaemia (B, F, J) anaemia and (C, G, K) weight loss and (D, H, L) survival. Groups consisted of 4 mice and the results are representative of 2 independent experiments. * indicates significant differences (p<0.05) between mice receiving WT and IL-10−/− CD4 T cells.
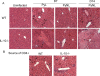
Figure 9. IL-10 ameliorates hepatic pathology during PyL and PyNL infection.
Liver pathology was examined (A) in WT and IL-10−/− mice that were either uninfected or had been infected with P. yoelii 7 days (PyL, PyNL) or 14 days (PyNL) previously, (B) on day 25 post-infection with PyNL in RAG-1−/− mice reconstituted with either WT or IL-10−/− CD4+ T cells prior to infection. Groups consisted of 4–5 mice and the slides shown are representative of mice from 2 independent experiments. Arrows highlight areas of interest: c = central vein periportal infiltration, pv = vessels packed with inflammatory cells, p = pigmented kupffer cells, n = necrosis, i = inflammation in parenchymia, pt = necrosis and gross infiltration in portal triad.
Similar articles
- Effects of CD4(+)CD25(+)Foxp3(+)regulatory T cells on early Plasmodium yoelii 17XL infection in BALB/c mice.
Chen G, Liu J, Wang QH, Wu Y, Feng H, Zheng W, Guo SY, Li DM, Wang JC, Cao YM. Chen G, et al. Parasitology. 2009 Sep;136(10):1107-20. doi: 10.1017/S0031182009990370. Epub 2009 Jul 2. Parasitology. 2009. PMID: 19573259 - Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta.
Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M. Scholzen A, et al. PLoS Pathog. 2009 Aug;5(8):e1000543. doi: 10.1371/journal.ppat.1000543. Epub 2009 Aug 14. PLoS Pathog. 2009. PMID: 19680449 Free PMC article. - Differential role of T regulatory and Th17 in Swiss mice infected with Plasmodium berghei ANKA and Plasmodium yoelii.
Keswani T, Bhattacharyya A. Keswani T, et al. Exp Parasitol. 2014 Jun;141:82-92. doi: 10.1016/j.exppara.2014.03.003. Epub 2014 Mar 24. Exp Parasitol. 2014. PMID: 24675415 - Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells.
Banham AH. Banham AH. Trends Immunol. 2006 Dec;27(12):541-4. doi: 10.1016/j.it.2006.10.002. Epub 2006 Oct 12. Trends Immunol. 2006. PMID: 17045841 Review. - T cell-derived IL-10 and its impact on the regulation of host responses during malaria.
Freitas do Rosario AP, Langhorne J. Freitas do Rosario AP, et al. Int J Parasitol. 2012 May 15;42(6):549-55. doi: 10.1016/j.ijpara.2012.03.010. Epub 2012 Apr 24. Int J Parasitol. 2012. PMID: 22549022 Review.
Cited by
- Immunologic Profiling of CSF in Subarachnoid Neurocysticercosis Reveals Specific Interleukin-10-Producing Cell Populations During Treatment.
Tang NL, Schaughency P, Gazzinelli-Guimaraes P, Lack J, Thumm L, Miltenberger E, Nash TE, Nutman TB, O'Connell EM. Tang NL, et al. Neurol Neuroimmunol Neuroinflamm. 2024 Nov;11(6):e200320. doi: 10.1212/NXI.0000000000200320. Epub 2024 Oct 30. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 39475624 Free PMC article. - NK cells contribute to the resolution of experimental malaria-associated acute respiratory distress syndrome after antimalarial treatment.
Pollenus E, Possemiers H, Knoops S, Prenen F, Vandermosten L, Pham TT, Buysrogge L, Matthys P, Van den Steen PE. Pollenus E, et al. Front Immunol. 2024 Sep 17;15:1433904. doi: 10.3389/fimmu.2024.1433904. eCollection 2024. Front Immunol. 2024. PMID: 39355242 Free PMC article. - Synergistic blockade of TIGIT and PD-L1 increases type-1 inflammation and improves parasite control during murine blood-stage Plasmodium yoelii non-lethal infection.
Dookie RS, Villegas-Mendez A, Cheeseman A, Jones AP, Barroso R, Barrett JR, Draper SJ, Janse CJ, Grogan JL, MacDonald AS, Couper KN. Dookie RS, et al. Infect Immun. 2024 Nov 12;92(11):e0034524. doi: 10.1128/iai.00345-24. Epub 2024 Sep 26. Infect Immun. 2024. PMID: 39324794 - CD4+ T cells display a spectrum of recall dynamics during re-infection with malaria parasites.
Lee HJ, Moreira ML, Li S, Asatsuma T, Williams CG, Skinner OP, Asad S, Bramhall M, Jiang Z, Liu Z, Kerr AS, Engel JA, Soon MSF, Straube J, Barrera I, Murray E, Chen F, Nideffer J, Jagannathan P, Haque A. Lee HJ, et al. Nat Commun. 2024 Jun 28;15(1):5497. doi: 10.1038/s41467-024-49879-6. Nat Commun. 2024. PMID: 38944658 Free PMC article. - Cytolytic circumsporozoite-specific memory CD4+ T cell clones are expanded during Plasmodium falciparum infection.
Furtado R, Paul M, Zhang J, Sung J, Karell P, Kim RS, Caillat-Zucman S, Liang L, Felgner P, Bauleni A, Gama S, Buchwald A, Taylor T, Seydel K, Laufer M, Delahaye F, Daily JP, Lauvau G. Furtado R, et al. Nat Commun. 2023 Nov 25;14(1):7726. doi: 10.1038/s41467-023-43376-y. Nat Commun. 2023. PMID: 38001069 Free PMC article.
References
- Engwerda C, Belnoue E, Gruner AC, Renia L. Experimental models of cerebral malaria. Curr Top Microbiol Immunol. 2005;297:103–143. - PubMed
- Omer FM, Kurtzhals JA, Riley EM. Maintaining the immunological balance in parasitic infections: a role for TGF-beta? Parasitol Today. 2000;16:18–23. - PubMed
- Omer FM, de Souza JB, Riley EM. Differential induction of TGF-beta regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J Immunol. 2003;171:5430–5436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials