Machine learning classifiers detect subtle field defects in eyes of HIV individuals - PubMed (original) (raw)
. 2007:105:111-8; discussion 119-20.
Affiliations
- PMID: 18427600
- PMCID: PMC2258123
Machine learning classifiers detect subtle field defects in eyes of HIV individuals
Igor Kozak et al. Trans Am Ophthalmol Soc. 2007.
Abstract
Purpose: To test the following hypotheses: (1) eyes from patients with human immunodeficiency virus (HIV) have retinal damage that causes subtle field defects, (2) sensitive machine learning classifiers (MLCs) can use these field defects to distinguish fields in HIV patients and normal subjects, and (3) the subtle field defects form meaningful patterns. We have applied supervised MLCs--support vector machine (SVM) and relevance vector machine (RVM)--to determine if visual fields in patients with HIV differ from normal visual fields in HIV-negative controls.
Methods: HIV-positive patients without visible retinopathy were divided into 2 groups: (1) 38 high-CD4 (H), 48.5 +/- 8.5 years, whose CD4 counts were never below 100; and (2) 35 low-CD4 (L), 46.1 +/- 8.5 years, whose CD4 counts were below 100 at least 6 months. The normal group (N) had 52 age-matched HIV-negative individuals, 46.3 +/- 7.8 years. Standard automated perimetry (SAP) with the 24-2 program was recorded from one eye per individual per group. SVM and RVM were trained and tested with cross-validation to distinguish H from N and L from N. Area under the receiver operating characteristic (AUROC) curve permitted comparison of performance of MLCs. Improvement in performance and identification of subsets of the most important features were sought with feature selection by backward elimination.
Results: SVM and RVM distinguished L from N (L: AUROC = 0.804, N: 0.500, P = .0002 with SVM and L: .800, P = .0002 with RVM) and H from N (H: 0.683, P = .022 with SVM and H: 0.670, P = .038 with RVM). With best-performing subsets derived by backward elimination, SVM and RVM each distinguished L from N (L: 0.843, P < .00005 with SVM and L: 0.870, P < .00005 with RVM) and H from N (H: 0.695, P = .015 with SVM and H: 0.726, P = .007 with RVM). The most important field locations in low-CD4 individuals were mostly superior near the blind spot. The location of important field locations was uncertain in high-CD4 eyes.
Conclusions: This study has confirmed that low-CD4 eyes have visual field defects and retinal damage. Ranking located important field locations superiorly near the blind spot, implying damage to the retina inferiorly near the optic disc. Though most fields appear normal in high-CD4 eyes, SVM and RVM were sufficiently sensitive to distinguish these eyes from normal eyes with SAP. The location of these defects is not yet defined. These results also validate the use of sensitive MLC techniques to uncover test differences not discernible by human experts.
Figures
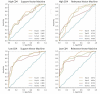
FIGURE 1
Receiver operating characteristic curves for support vector machine, relevance vector machine, high CD4, and low CD4. Within each graph are curves generated for machine learning classifiers trained to distinguish HIV eyes from normal eyes using all 52 field locations, the subset with peak performance, and the 10-location feature set; the chance curve is the attempt to learn classes with equivalent data.
FIGURE 2
Performance curves measuring area under the receiver operating characteristic (AUROC) for each size subset of near optimal combinations of features generated by backward elimination from all 52 features down to 1 feature. The upper, dashed blue curve averages curves derived from standard backward elimination, which graphs the AUROC of the selected set for each set size. The peak (blue arrow) is the subset size with the best performance. The lower, continuous red curve averages the results after the extra step of external cross-validation to give a more conservative estimate of performance at each step of backward elimination. In the low-CD4 eyes, performance did not improve with extra-validated feature sets larger than the set with 4 dependent features. In high-CD4 eyes, small subsets performed poorly and performance increased up to the full 52-location feature set.
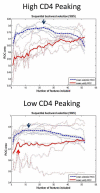
FIGURE 3
Plot of the 10 best features in combination, from backward elimination. In the high-CD4 eyes, small sets did not perform well, indicating uncertain distribution of the top features (small numbers). In the low-CD4 eyes, feature sets larger than 4 did not improve performance, indicating the top 4 field locations (large numbers) were strong predictors of HIV.
Similar articles
- Pattern recognition can detect subtle field defects in eyes of HIV individuals without retinitis under HAART.
Goldbaum MH, Kozak I, Hao J, Sample PA, Lee T, Grant I, Freeman WR. Goldbaum MH, et al. Graefes Arch Clin Exp Ophthalmol. 2011 Apr;249(4):491-8. doi: 10.1007/s00417-010-1511-x. Epub 2010 Sep 24. Graefes Arch Clin Exp Ophthalmol. 2011. PMID: 20865422 Free PMC article. - Analysis with support vector machine shows HIV-positive subjects without infectious retinitis have mfERG deficiencies compared to normal eyes.
Goldbaum MH, Falkenstein I, Kozak I, Hao J, Bartsch DU, Sejnowski T, Freeman WR. Goldbaum MH, et al. Trans Am Ophthalmol Soc. 2008;106:196-204; discussion 204-5. Trans Am Ophthalmol Soc. 2008. PMID: 19277235 Free PMC article. - Sensitivity and specificity of machine learning classifiers for glaucoma diagnosis using Spectral Domain OCT and standard automated perimetry.
Silva FR, Vidotti VG, Cremasco F, Dias M, Gomi ES, Costa VP. Silva FR, et al. Arq Bras Oftalmol. 2013 May-Jun;76(3):170-4. doi: 10.1590/s0004-27492013000300008. Arq Bras Oftalmol. 2013. PMID: 23929078 - Unsupervised learning with independent component analysis can identify patterns of glaucomatous visual field defects.
Goldbaum MH. Goldbaum MH. Trans Am Ophthalmol Soc. 2005;103:270-80. Trans Am Ophthalmol Soc. 2005. PMID: 17057807 Free PMC article. - Can frequency-doubling technology and short-wavelength automated perimetries detect visual field defects before standard automated perimetry in patients with preperimetric glaucoma?
Ferreras A, Polo V, Larrosa JM, Pablo LE, Pajarin AB, Pueyo V, Honrubia FM. Ferreras A, et al. J Glaucoma. 2007 Jun-Jul;16(4):372-83. doi: 10.1097/IJG.0b013e31803bbb17. J Glaucoma. 2007. PMID: 17571000
Cited by
- Retinal Thickening and Photoreceptor Loss in HIV Eyes without Retinitis.
Arcinue CA, Bartsch DU, El-Emam SY, Ma F, Doede A, Sharpsten L, Gomez ML, Freeman WR. Arcinue CA, et al. PLoS One. 2015 Aug 5;10(8):e0132996. doi: 10.1371/journal.pone.0132996. eCollection 2015. PLoS One. 2015. PMID: 26244973 Free PMC article. - Pattern recognition can detect subtle field defects in eyes of HIV individuals without retinitis under HAART.
Goldbaum MH, Kozak I, Hao J, Sample PA, Lee T, Grant I, Freeman WR. Goldbaum MH, et al. Graefes Arch Clin Exp Ophthalmol. 2011 Apr;249(4):491-8. doi: 10.1007/s00417-010-1511-x. Epub 2010 Sep 24. Graefes Arch Clin Exp Ophthalmol. 2011. PMID: 20865422 Free PMC article. - Human immunodeficiency virus and its effects on the visual system.
Stewart MW. Stewart MW. Infect Dis Rep. 2012 Mar 8;4(1):e25. doi: 10.4081/idr.2012.e25. eCollection 2012 Jan 2. Infect Dis Rep. 2012. PMID: 24470932 Free PMC article. Review. - A degenerative retinal process in HIV-associated non-infectious retinopathy.
Kozak I, Sasik R, Freeman WR, Sprague LJ, Gomez ML, Cheng L, El-Emam S, Mojana F, Bartsch DU, Bosten J, Ayyagari R, Hardiman G. Kozak I, et al. PLoS One. 2013 Sep 17;8(9):e74712. doi: 10.1371/journal.pone.0074712. eCollection 2013. PLoS One. 2013. PMID: 24069333 Free PMC article. - Association between retinal nerve fiber layer thickness and abnormalities of vision in people with human immunodeficiency virus infection.
Kalyani PS, Holland GN, Fawzi AA, Arantes TE, Yu F, Sadun AA; Studies of the Ocular Complications of AIDS Research Group. Kalyani PS, et al. Am J Ophthalmol. 2012 Apr;153(4):734-42, 742.e1. doi: 10.1016/j.ajo.2011.09.019. Epub 2012 Jan 15. Am J Ophthalmol. 2012. PMID: 22245459 Free PMC article.
References
- Goldbaum MH, Sample PA, White H, et al. Interpretation of automated perimetry for glaucoma by neural network. Invest Ophthalmol Vis Sci. 1994;35:3362–3373. - PubMed
- Mutlukan E, Keating K. Visual field interpretation with a personal computer based neural network. Eye. 1994;8:321–323. - PubMed
- Brigatti L, Nouri-Mahdavi K, Weitzman M, Caprioli J. Automatic detection of glaucomatous visual field progression with neural networks. Arch Ophthalmol. 1997;115:725–728. - PubMed
- Brigatti L, Hoffman BA, Caprioli J. Neural networks to identify glaucoma with structural and functional measurements. Am J Ophthalmol. 1996;121:511–521. - PubMed
Publication types
MeSH terms
Grants and funding
- EY 13928/EY/NEI NIH HHS/United States
- EY07366/EY/NEI NIH HHS/United States
- EY08308/EY/NEI NIH HHS/United States
- R21 EY013928/EY/NEI NIH HHS/United States
- R33 EY013928/EY/NEI NIH HHS/United States
- R01 EY007366/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous