Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism - PubMed (original) (raw)
Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism
Vasilis Androutsopoulos et al. Breast Cancer Res. 2008.
Abstract
Introduction: The natural product eupatorin has been reported to have antiproliferative activity in tumour cell lines, but the exact mechanism is unclear. The cytochromes P450 CYP1B1, CYP1A1, and CYP1A2 have been shown to participate in the activation of various xenobiotics, compounds derived from the diet as well as chemotherapeutic drugs. CYP1B1 and CYP1A1 have also been proposed as targets for cancer chemotherapy for their differential and selective overexpression in tumour cells. In this study, we aimed to identify a possible mechanism of action for the antiproliferative effect of eupatorin, which can be attributed to CYP1 family-mediated metabolism.
Methods: The study focuses on the antiproliferative action of eupatorin on the human breast carcinoma cell line MDA-MB-468 and on a cell line derived from normal mammary tissue, MCF-10A. The cytotoxicity of the flavone, its effect on the cell cycle of the abovementioned cell lines, and its metabolism by CYP1 family enzymes were examined.
Results: Eupatorin showed a dose-dependent inhibitory effect of cell growth on MDA-MB-468 cells with a submicromolar median inhibition concentration (IC50) whereas the IC50 of this compound in MCF-10A cells was considerably higher. The antiproliferative effect, as measured by EROD (ethoxyresorufin-O-deethylase) assay and Western immunoblotting, was attributed mainly to CYP1A1 expression in MDA-MB-468 cells but not in MCF-10A cells. Moreover, CYP1 family enzymes were shown to metabolise eupatorin in vitro to the flavone cirsiliol and two other unidentified metabolites. Metabolism of eupatorin was also detected in MDA-MB-468 cell cultures, whereas metabolism by MCF-10A cells was negligible. Eupatorin was further shown to arrest the cell cycle of the CYP1-expressing cell line MDA-MB-468 in G2/M phase, whereas no effect was observed in MCF-10A cells, which do not express CYP1 enzymes. The effect of eupatorin on the MDA-MB-468 cell cycle could be reversed by co-application of the CYP1 inhibitor acacetin.
Conclusion: The flavone eupatorin is selectively activated in breast cancer cells, but not in normal breast cells, due to CYP1 family metabolism. This provides a basis for selectivity which is desired against breast tumour cells. In this sense, eupatorin is shown by this study to be a very promising chemopreventative candidate that should be examined further in an in vivo study.
Figures
Figure 1
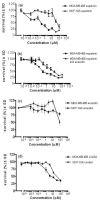
MTT cell proliferation assays. (a) Cytotoxicity of eupatorin in MDA-MB-468 cells and MCF-10A cells. (b) Decrease of eupatorin cytotoxicity in MDA-MB-468 cells after addition of acacetin. (c) Cytotoxicity of acacetin in MDA-MB-468 cells and MCF-10A cells. (d) Cytotoxicity of cirsiliol in MDA-MB-468 cells and MCF-10A cells. Cells were plated into 96-well plates and treated with 10-3 to 100 μM eupatorin, acacetin, or cirsiliol (as described in Materials and methods) and allowed to grow for 96 hours. For the inhibition experiment, acacetin was used at a final concentration of 1.5 μM. Error bars represent mean ± standard deviation (SD) for n = 4 determinations. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Figure 2
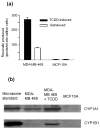
CYP1 enzyme expression in MDA-MB-468 and MCF-10A cells. (a) EROD activity of MDA-MB-468 and MCF-10A cells. Cells were seeded at a density of 5 × 104 cells per millilitre in 24-well plates and left to grow for 48 hours. EROD activity was measured as described in Materials and methods. Error bars represent mean ± standard deviation for n = 4 determinations. (b) Selective and inducible CYP1A and CYP1B1 expression in MDA-MB-468 cells. Lysates were probed with anti-CYP1A and anti-CYP1B1 antibodies from Gentest Corporation (now part of BD Biosciences) and Auvation Limited. Lane 1: Recombinant CYP1A1 (top, 0.2 μg) or CYP1B1 (bottom, 0.4 μg) used as positive control. Lane 2: MDA-MB-468 cells. Lane 3: MDA-MB-468 cells treated with 10 nM TCDD for 24 hours. Lane 4: MCF-10A cells. Experiments were performed in duplicate. EROD, ethoxyresorufin-_O_-deethylase; TCDD, 2,3,7,8-tetrachlorodibenzo-_p_-dioxin.
Figure 3
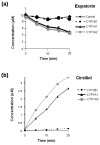
Rate of metabolism of eupatorin (10 μM) by recombinant microsomes expressing CYP1A1, CYP1A2, or CYP1B1. (a) Disappearance of eupatorin over time by CYP1 family enzymes. (b) Formation of the metabolite cirsiliol over a 20-minute time period. Experiments were performed in duplicate as described in Materials and methods. Error bars represent mean ± minimum or maximum values for n = 2 determinations.
Figure 4
Metabolic profile of eupatorin (10 μM) metabolism by CYP1 family enzymes and identification of cirsiliol as the primary metabolite. (a) Typical high-pressure liquid chromatography (HPLC) traces of 20-minute incubation of CYP1 enzymes with eupatorin. (b) Expansion of (a) showing metabolites E2 and E3. (c) Co-elution studies of eupatorin with cirsiliol. A 20-minute CYP1B1 incubate of eupatorin was spiked with cirsiliol (0.2 μM). Reaction mixtures contained eupatorin, NADPH (nicotinamide adenine dinucleotide phosphate), and recombinant microsomes purchased from Gentest Corporation (now part of BD Biosciences). Reactions were terminated by the addition of 1% acetic acid in methanol. (d) HPLC trace of metabolism of eupatorin in MDA-MB-468 cells. Samples were analysed by HPLC using a UV detector at 350 nm. Experiments were performed in triplicate. A350: absorption of light at wavelength 350 nm. AU: arbitrary units.
Figure 5
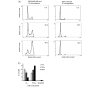
Eupatorin causes G2/M arrest in MDA-MB-468 cells, whereas this effect is not observed in MCF-10A cells. (a) Flow cytometric DNA analysis of MDA-MB-468 and MCF-10A cells treated with 10 μM eupatorin. (b) Percentage of MDA-MB-468 cells in G1, S+G2/M, and sub-G1 phases of the cell cycle. Cells were plated in 24-well plates and left to grow for 48 hours. Eupatorin was incubated with the cells for 30 and 48 hours, and 0.1% dimethylsulfoxide was used as a control. The cells were stained with propidium iodide and analysed using a Beckman Coulter flow cytometer as described in Materials and methods. Error bars represent mean ± standard deviation for n = 3 determinations.
Figure 6
G2/M arrest of MDA-MB-468 caused by eupatorin is due in part to metabolism to cirsiliol and can be reversed by acacetin. (a) Cell cycle analysis of MDA-MB-468 cells co-treated with 10 μM eupatorin and 1.5 μM acacetin for 48 hours. Histograms are one trace of three independent experiments. (b) Cell cycle profile of MDA-MB-468 cells treated with 10 μM cirsiliol for 48 hours. The experiment was performed in duplicate.
Figure 7
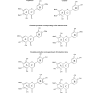
Chemical structures of eupatorin and cirsiliol and possible structures of the metabolites E2 and E3.
Similar articles
- CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells.
Androutsopoulos VP, Ruparelia K, Arroo RR, Tsatsakis AM, Spandidos DA. Androutsopoulos VP, et al. Toxicology. 2009 Oct 29;264(3):162-70. doi: 10.1016/j.tox.2009.07.023. Epub 2009 Aug 8. Toxicology. 2009. PMID: 19666078 - Comparative CYP1A1 and CYP1B1 substrate and inhibitor profile of dietary flavonoids.
Androutsopoulos VP, Papakyriakou A, Vourloumis D, Spandidos DA. Androutsopoulos VP, et al. Bioorg Med Chem. 2011 May 1;19(9):2842-9. doi: 10.1016/j.bmc.2011.03.042. Epub 2011 Mar 24. Bioorg Med Chem. 2011. PMID: 21482471 - Tangeretin inhibits the proliferation of human breast cancer cells via CYP1A1/CYP1B1 enzyme induction and CYP1A1/CYP1B1-mediated metabolism to the product 4' hydroxy tangeretin.
Surichan S, Arroo RR, Tsatsakis AM, Androutsopoulos VP. Surichan S, et al. Toxicol In Vitro. 2018 Aug;50:274-284. doi: 10.1016/j.tiv.2018.04.001. Epub 2018 Apr 4. Toxicol In Vitro. 2018. PMID: 29626627 - Cytochrome P450 1 family and cancers.
Go RE, Hwang KA, Choi KC. Go RE, et al. J Steroid Biochem Mol Biol. 2015 Mar;147:24-30. doi: 10.1016/j.jsbmb.2014.11.003. Epub 2014 Nov 6. J Steroid Biochem Mol Biol. 2015. PMID: 25448748 Review. - Dietary flavonoids in cancer therapy and prevention: substrates and inhibitors of cytochrome P450 CYP1 enzymes.
Androutsopoulos VP, Papakyriakou A, Vourloumis D, Tsatsakis AM, Spandidos DA. Androutsopoulos VP, et al. Pharmacol Ther. 2010 Apr;126(1):9-20. doi: 10.1016/j.pharmthera.2010.01.009. Epub 2010 Feb 11. Pharmacol Ther. 2010. PMID: 20153368 Review.
Cited by
- Characterization of the interaction between eupatorin and bovine serum albumin by spectroscopic and molecular modeling methods.
Xu H, Yao N, Xu H, Wang T, Li G, Li Z. Xu H, et al. Int J Mol Sci. 2013 Jul 9;14(7):14185-203. doi: 10.3390/ijms140714185. Int J Mol Sci. 2013. PMID: 23839090 Free PMC article. - Artemisia annua, a Traditional Plant Brought to Light.
Septembre-Malaterre A, Lalarizo Rakoto M, Marodon C, Bedoui Y, Nakab J, Simon E, Hoarau L, Savriama S, Strasberg D, Guiraud P, Selambarom J, Gasque P. Septembre-Malaterre A, et al. Int J Mol Sci. 2020 Jul 15;21(14):4986. doi: 10.3390/ijms21144986. Int J Mol Sci. 2020. PMID: 32679734 Free PMC article. Review. - Antitumour activity of the novel flavonoid Oncamex in preclinical breast cancer models.
Martínez-Pérez C, Ward C, Turnbull AK, Mullen P, Cook G, Meehan J, Jarman EJ, Thomson PI, Campbell CJ, McPhail D, Harrison DJ, Langdon SP. Martínez-Pérez C, et al. Br J Cancer. 2016 Apr 12;114(8):905-16. doi: 10.1038/bjc.2016.6. Epub 2016 Mar 31. Br J Cancer. 2016. PMID: 27031849 Free PMC article. - CYP1-Activation and Anticancer Properties of Synthetic Methoxylated Resveratrol Analogues.
Ruparelia KC, Zeka K, Beresford KJM, Wilsher NE, Potter GA, Androutsopoulos VP, Brucoli F, Arroo RRJ. Ruparelia KC, et al. Molecules. 2024 Jan 15;29(2):423. doi: 10.3390/molecules29020423. Molecules. 2024. PMID: 38257336 Free PMC article. - Anthricin Isolated from Anthriscus sylvestris (L.) Hoffm. Inhibits the Growth of Breast Cancer Cells by Inhibiting Akt/mTOR Signaling, and Its Apoptotic Effects Are Enhanced by Autophagy Inhibition.
Jung CH, Kim H, Ahn J, Jung SK, Um MY, Son KH, Kim TW, Ha TY. Jung CH, et al. Evid Based Complement Alternat Med. 2013;2013:385219. doi: 10.1155/2013/385219. Epub 2013 May 29. Evid Based Complement Alternat Med. 2013. PMID: 23818925 Free PMC article.
References
- Draper L. Breast cancer: trends, risks, treatments and effects. AAOHN J. 2006;54:445–451. - PubMed
- Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–1284. - PubMed
- Tezuka Y, Stampoulis P, Banskota AH, Awale S, Tran KQ, Saiki I, Kadota S. Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. Chem Pharm Bull. 2000;48:1711–1719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous