DNA strand breaks, neurodegeneration and aging in the brain - PubMed (original) (raw)
Review
DNA strand breaks, neurodegeneration and aging in the brain
Sachin Katyal et al. Mech Ageing Dev. 2008 Jul-Aug.
Abstract
Defective responses to DNA single- or double-strand breaks can result in neurological disease, underscoring the critical importance of DNA repair for neural homeostasis. Human DNA repair-deficient syndromes are generally congenital, in which brain pathology reflects the consequences of developmentally incurred DNA damage. Although, it is unclear to what degree DNA strand-break repair defects in mature neural cells contributes to disease pathology. However, DNA single-strand breaks are a relatively common lesion which if not repaired can impact cells via interference with transcription. Thus, this lesion, and probably to a lesser extent DNA double-strand breaks, may be particularly relevant to aging in the neural cell population. In this review we will examine the consequences of defective DNA strand-break repair towards homeostasis in the brain. Further, we also consider the utility of mouse models as reagents to understand the connection between DNA strand breaks and aging in the brain.
Figures
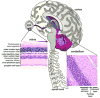
Figure 1. The adult mammalian CNS
The mature nervous system contains a myriad of different cell types and tissues. DNA repair processes impact substantially during neural development leading to defective neurogenesis and development. However, less is known regarding the requirement for DNA repair processes in mature neural cell. Inset panels are hematoxylin and eosin stained retinal and cerebellar sections that show cell organization in these tissues. The retina is laminar in nature and cell-types are stratified into three distinct nuclear layers: outer, inner and ganglion. The outer nuclear layer contains the photoreceptor (rods and cones) neurons. The inner nuclear layer contains various signal processing cell types: bipolar, horizontal, amacrine, interplexiform and the Müller glia. The ganglion cells carry the visual signal via its axons through the optic nerve and project onto the brain. The cerebellum is stratified into three primary layers: inner granule cell layer, the Purkinje cell layer and the molecular layer. Excitory sensory signals originating from the cerebellum are ultimately transmitted through granule-Purkinje synapses and out of the cerebellum through Purkinje neuron axons to affect normal control of movement.
Similar articles
- A nervous predisposition to unrepaired DNA double strand breaks.
Reynolds JJ, Stewart GS. Reynolds JJ, et al. DNA Repair (Amst). 2013 Aug;12(8):588-99. doi: 10.1016/j.dnarep.2013.04.011. Epub 2013 May 17. DNA Repair (Amst). 2013. PMID: 23684796 Review. - DNA repair deficiency in neurodegeneration.
Jeppesen DK, Bohr VA, Stevnsner T. Jeppesen DK, et al. Prog Neurobiol. 2011 Jul;94(2):166-200. doi: 10.1016/j.pneurobio.2011.04.013. Epub 2011 Apr 30. Prog Neurobiol. 2011. PMID: 21550379 Free PMC article. Review. - DNA repair deficiency and neurodegeneration.
Katyal S, McKinnon PJ. Katyal S, et al. Cell Cycle. 2007 Oct 1;6(19):2360-5. doi: 10.4161/cc.6.19.4757. Epub 2007 Jul 18. Cell Cycle. 2007. PMID: 17700067 Review. - DNA strand break repair and neurodegeneration.
Rulten SL, Caldecott KW. Rulten SL, et al. DNA Repair (Amst). 2013 Aug;12(8):558-67. doi: 10.1016/j.dnarep.2013.04.008. Epub 2013 May 24. DNA Repair (Amst). 2013. PMID: 23712058 Review. - A single strand that links multiple neuropathologies in human disease.
Reynolds JJ, Stewart GS. Reynolds JJ, et al. Brain. 2013 Jan;136(Pt 1):14-27. doi: 10.1093/brain/aws310. Brain. 2013. PMID: 23365091 Review.
Cited by
- Dietary Plant Polyphenols as the Potential Drugs in Neurodegenerative Diseases: Current Evidence, Advances, and Opportunities.
Yan L, Guo MS, Zhang Y, Yu L, Wu JM, Tang Y, Ai W, Zhu FD, Law BY, Chen Q, Yu CL, Wong VK, Li H, Li M, Zhou XG, Qin DL, Wu AG. Yan L, et al. Oxid Med Cell Longev. 2022 Feb 21;2022:5288698. doi: 10.1155/2022/5288698. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35237381 Free PMC article. Review. - Exonic Variants in Aging-Related Genes Are Predictive of Phenotypic Aging Status.
Breitbach ME, Greenspan S, Resnick NM, Perera S, Gurkar AU, Absher D, Levine AS. Breitbach ME, et al. Front Genet. 2019 Dec 19;10:1277. doi: 10.3389/fgene.2019.01277. eCollection 2019. Front Genet. 2019. PMID: 31921313 Free PMC article. - Effects of DSP4 on the noradrenergic phenotypes and its potential molecular mechanisms in SH-SY5Y cells.
Wang Y, Musich PR, Serrano MA, Zou Y, Zhang J, Zhu MY. Wang Y, et al. Neurotox Res. 2014 Feb;25(2):193-207. doi: 10.1007/s12640-013-9421-4. Epub 2013 Aug 31. Neurotox Res. 2014. PMID: 23996700 Free PMC article. - Healthy brain aging and delayed dementia in Texas rural elderly.
Basu T, Sehar U, Malhotra K, Culberson J, Khan H, Morton H, Orlov E, Brownell M, Reddy PH. Basu T, et al. Ageing Res Rev. 2023 Nov;91:102047. doi: 10.1016/j.arr.2023.102047. Epub 2023 Aug 29. Ageing Res Rev. 2023. PMID: 37652312 Free PMC article. Review.
References
- Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature. 2006;443(7112):713–716. - PubMed
- Ahnesorg P, Smith P, Jackson SP. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 2006;124(2):301–313. - PubMed
- Andres SN, Modesti M, Tsai CJ, Chu G, Junop MS. Crystal structure of human XLF: a twist in nonhomologous DNA end-joining. Molecular cell. 2007;28(6):1093–1101. - PubMed
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86(1):159–171. - PubMed
- Barnes DE, Stamp G, Rosewell I, Denzel A, Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol. 1998;8(25):1395–1398. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical