Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness - PubMed (original) (raw)
Comparative Study
Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness
Núria de la Iglesia et al. J Neurosci. 2008.
Abstract
Inactivation of the tumor suppressor phosphatase and tensin homolog (mutated in multiple advanced cancers 1) (PTEN) is recognized as a major event in the pathogenesis of the brain tumor glioblastoma. However, the mechanisms by which PTEN loss specifically impacts the malignant behavior of glioblastoma cells, including their proliferation and propensity for invasiveness, remain poorly understood. Genetic studies suggest that the transcription factor signal transducers and activators of transcription 3 (STAT3) harbors a PTEN-regulated tumor suppressive function in mouse astrocytes. Here, we report that STAT3 plays a critical tumor suppressive role in PTEN-deficient human glioblastoma cells. Endogenous STAT3 signaling is specifically inhibited in PTEN-deficient glioblastoma cells. Strikingly, reactivation of STAT3 in PTEN-deficient glioblastoma cells inhibits their proliferation, invasiveness, and ability to spread on myelin. We also identify the chemokine interleukin 8 (IL8) as a novel target gene of STAT3 in human glioblastoma cells. Activated STAT3 occupies the endogenous IL8 promoter and directly represses IL8 transcription. Consistent with these results, IL8 is upregulated in PTEN-deficient human glioblastoma tumors. Importantly, IL8 repression mediates STAT3 inhibition of glioblastoma cell proliferation, invasiveness, and spreading on myelin. Collectively, our findings uncover a novel link between STAT3 and IL8, the deregulation of which plays a key role in the malignant behavior of PTEN-deficient glioblastoma cells. These studies suggest that STAT3 activation or IL8 inhibition may have potential in patient-tailored treatment of PTEN-deficient brain tumors.
Figures
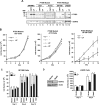
Figure 1.
Endogenous STAT3 inhibits proliferation of wild-type PTEN-expressing glioblastoma cells. A, Lysates of glioblastoma cells that express wild-type PTEN (SF188 and LN229) or glioblastoma cells that harbor PTEN mutations (U87 and A172) were immunoblotted with STAT3 and Tyr 705-phosphorylated STAT3 (pYS3) antibodies. Cells were serum starved for 24 h and then treated with 100 ng/ml CNTF or 10 ng/ml LIF for 15 min. LIF treatment robustly induced STAT3 tyrosine phosphorylation in control SKNMC neuroblastoma cells and in the wild-type PTEN-expressing glioblastoma cells but failed to effectively induce the STAT3 phosphorylation in the PTEN-deficient glioblastoma cells. B, Growth curves of PTEN-mutant (U87 and A172) and PTEN-expressing (SF188) glioblastoma cells that were treated with LIF (10 ng/ml daily) or left untreated. LIF did not affect cell population growth of U87 (representative experiment shown) or A172 cells (two representative experiments shown) but significantly reduced SF188 cell population growth (n = 3; paired t test, *p < 0.05). C, Cell population growth of SF188 cells infected with a retrovirus containing an IRES-GFP cassette alone or that also encodes wild-type STAT3 or a dominant-negative STAT3 (S3D). LIF (10 ng/ml daily) led to a significant reduction in population growth of control vector or wild-type STAT3-expressing cells (n = 3; ANOVA, *p < 0.05, ¶p < 0.01, §p < 0.001) but not in S3D-expressing SF188 cells. D, Left, Lysates of LN229 cells infected with either control lentivirus or lentivirus encoding a small interfering hairpin RNA directed at STAT3 and selected with puromycin were immunoblotted with an STAT3 antibody. Actin was used as loading control. The Stat3i hairpin RNA induced knockdown of endogenous human STAT3. Right, Time course of the growth of LN229 cells infected with control lentivirus or lentivirus encoding Stat3i and selected with puromycin. LIF (10 ng/ml daily) led to a significant reduction in growth in control-infected (n = 3; ANOVA, *p < 0.05) but not Stat3i hairpin RNA-expressing LN229 cells.
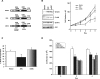
Figure 2.
Expression of activated STAT3 inhibits proliferation PTEN-deficient glioblastoma cells. A, Schematic of activated STAT3 (S3C) or a DNA-binding mutant version of S3C (mS3C). B, Left, Lysates of U87 glioblastoma cells infected with a control retrovirus containing an IRES-GFP cassette or retroviruses that also encode FLAG-tagged S3C or mS3C were immunoblotted with STAT3 and FLAG antibodies and antibodies to STAT1 or Akt as loading controls. Right, Cell population growth curves of U87 glioblastoma cells. S3C-expressing U87 cells proliferated significantly more slowly than mS3C-expressing U87 cells or those infected with control virus (n = 3; ANOVA, *p < 0.05). C, BrdU labeling of U87 glioblastoma stable cells measured as a percentage of the total number of cells. Incorporation of BrdU was significantly reduced in U87-S3C cells compared with both vector and mS3C (n = 4; ANOVA, *p < 0.05). D, Cell population growth curves of PTEN-deficient U87 cells or isogenic PTEN-expressing U87 cells infected with the S3C or control retrovirus. Expression of S3C significantly reduced the proliferation of U87 but not of isogenic PTEN-expressing U87 glioblastoma cells (representative experiment of 3 independent experiments performed in triplicate; ANOVA, *p < 0.001, **p < 0.0001).
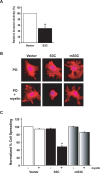
Figure 3.
STAT3 decreases glioblastoma cell invasiveness and spreading on myelin. A, Matrigel invasion assay of U87 glioblastoma stable cell lines. Significantly more U87-vector cells invaded the matrigel substrate than U87-S3C cells (n = 3; t test, *p < 0.05). STAT3 inhibition of U87 glioblastoma cell invasiveness was not secondary to the effect of activated STAT3 on U87 cell proliferation, because the invasive potential of these cells was measured at a time (22 h after plating) before the inhibitory effects of activated STAT3 on U87 cell proliferation (Fig. 2_B_). Equivalent numbers of NIH3T3 cells failed to invade the matrigel (data not shown). B, Phalloidin red staining of actin stress fibers of stable U87 glioblastoma cells plated onto coverslips coated with either polyornithine (PO) or polyornithine together with myelin (20 μg/ml). U87-S3C glioblastoma cells failed to spread on myelin (middle bottom panel), compared with a spread appearance on a polyornithine control substrate (middle top panel). U87-vector and U87-mS3C glioblastoma cells spread and formed stress fibers on myelin (right and left bottom panels). C, Quantification of cell spreading of U87 stable cells on myelin. Significantly fewer U87-S3C glioblastoma cells spread on myelin compared with U87-vector glioblastoma cells (n = 3; ANOVA, *p < 0.0001). Cells were counted in a blinded manner in three independent experiments, and the percentage of spreading was determined by calculating the number of spread cells over the total number of cells.
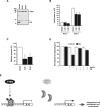
Figure 4.
IL8 is a direct STAT3-repressed target gene. A, Diagrammatic representation of the top 50 genes in three independent microarray analyses of control or S3C-expressing U87 cells, ranked according to fold change, that were repressed or induced after S3C expression. B, Top 10 repressed (left) or induced (right) genes after S3C expression. The fold change is indicated, and GenBank numbers are in parenthesis. C, Top, Immunoblotting of lysates of human glioblastoma samples (GBM) with an IL8 antibody. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control [GAPDH immunoblotting of some of the GBM samples is also shown by de la Iglesia et al. (2008)]. Eight of the tumors (#4, 8, 11, 13, 16, 19, 24, and 25) displayed very high levels of IL8. The majority of these tumors (6 of 8) also had low PTEN levels (de la Iglesia et al., 2008). Bottom, Fisher's exact test of IL8-positive and IL8-negative tumors revealed a strong correlation between IL8 expression and low PTEN levels [odds ratio (OR), 14; p < 0.01]. D, Left, Northern analysis of control vector-infected U87 glioblastoma cells or those expressing S3C using an IL8 probe. 18S RNA levels indicate equal loading. Middle, Immunoblotting of lysates of control U87 glioblastoma cells or those expressing S3C or mS3C with an IL8 antibody. The asterisk indicates a nonspecific band. Right, Sandwich ELISA chemiluminescent analysis of medium from the control U87 glioblastoma cells or those expressing S3C using two antibodies specific for human IL8 (R&D Systems). Shown are mean IL8 concentration ± SEM (pg of IL8/mg of cell protein; n = 3; t test, *p < 0.01). E, STAT3 represses the IL8 promoter. _Pten_−/− astrocytes were transfected with increasing amounts of an expression plasmid encoding S3C or a control plasmid together with an IL8 promoter-controlled luciferase reporter gene and a renilla expression plasmid and subjected to dual luciferase assay 48 h after transfection. Activated STAT3 significantly reduced IL8 promoter activity (n = 3; ANOVA, *p < 0.0001). V, Vector. F, Chromatin immunoprecipitation analysis at the endogenous IL8 promoter in S3C-expressing U87 glioblastoma cells using two different STAT3 antibodies (m, mouse; r, rabbit). Mouse and rabbit HA antibodies were used as controls. Negative controls for the PCR were performed with primers for the E-cadherin gene. STAT3 directly bound to the endogenous IL8 gene.
Figure 5.
IL8 promotes glioblastoma cell proliferation, invasiveness, and spreading on myelin. A, Lysates of U87 cells infected with IL8 RNAi-encoding lentiviruses (IL8i 1 or IL8i 2) or an empty vector and selected with puromycin were immunoblotted with IL8 and actin antibodies. The asterisk indicates a nonspecific band. B, Cell population growth curves of IL8 knockdown or vector-control U87 glioblastoma cells. IL8 knockdown suppressed glioblastoma cell population growth (n = 3; ANOVA, *p < 0.001). C, Matrigel invasion assay of U87 glioblastoma cells infected with control or IL8 RNAi lentiviruses. Significantly more U87-vector glioblastoma cells invaded the matrigel substrate than U87-IL8i 1 or U87-IL8i 2 glioblastoma cells (ANOVA, *p < 0.01). D, IL8 addition rescues STAT3 suppression of glioma cell spreading on myelin. Quantification of cell spreading shows that, in the presence of IL8, significantly more U87-S3C cells spread on myelin compared with untreated U87-S3C cells plated on myelin (n = 3; ANOVA, *p < 0.0005). E, Model of the PTEN-regulated STAT3–IL8 signaling link in human glioblastoma cells. Left, In the presence of the tumor suppressor PTEN, STAT3 is activated by phosphorylation, binds to the IL8 promoter, and represses IL8 gene expression. Right, STAT3 is inhibited after PTEN deficiency, thus relieving repression of the IL8 gene. Upregulation of IL8 promotes glioblastoma cell proliferation and invasiveness.
Similar articles
- STAT3-iNOS Signaling Mediates EGFRvIII-Induced Glial Proliferation and Transformation.
Puram SV, Yeung CM, Jahani-Asl A, Lin C, de la Iglesia N, Konopka G, Jackson-Grusby L, Bonni A. Puram SV, et al. J Neurosci. 2012 Jun 6;32(23):7806-18. doi: 10.1523/JNEUROSCI.3243-11.2012. J Neurosci. 2012. PMID: 22674257 Free PMC article. - Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells.
Senft C, Priester M, Polacin M, Schröder K, Seifert V, Kögel D, Weissenberger J. Senft C, et al. J Neurooncol. 2011 Feb;101(3):393-403. doi: 10.1007/s11060-010-0273-y. Epub 2010 Jun 30. J Neurooncol. 2011. PMID: 20589525 - ARL4C stabilized by AKT/mTOR pathway promotes the invasion of PTEN-deficient primary human glioblastoma.
Chen Q, Weng HY, Tang XP, Lin Y, Yuan Y, Li Q, Tang Z, Wu HB, Yang S, Li Y, Zhao XL, Fu WJ, Niu Q, Feng H, Zhang X, Wang Y, Bian XW, Yao XH. Chen Q, et al. J Pathol. 2019 Feb;247(2):266-278. doi: 10.1002/path.5189. Epub 2018 Dec 24. J Pathol. 2019. PMID: 30357833 - PTEN signaling pathways in glioblastoma.
Koul D. Koul D. Cancer Biol Ther. 2008 Sep;7(9):1321-5. doi: 10.4161/cbt.7.9.6954. Epub 2008 Sep 8. Cancer Biol Ther. 2008. PMID: 18836294 Review. - STAT3 regulation of glioblastoma pathogenesis.
de la Iglesia N, Puram SV, Bonni A. de la Iglesia N, et al. Curr Mol Med. 2009 Jun;9(5):580-90. doi: 10.2174/156652409788488739. Curr Mol Med. 2009. PMID: 19601808 Free PMC article. Review.
Cited by
- Mutant tristetraprolin: a potent inhibitor of malignant glioma cell growth.
Suswam EA, Shacka JJ, Walker K, Lu L, Li X, Si Y, Zhang X, Zheng L, Nabors LB, Cao H, King PH. Suswam EA, et al. J Neurooncol. 2013 Jun;113(2):195-205. doi: 10.1007/s11060-013-1112-8. Epub 2013 Mar 25. J Neurooncol. 2013. PMID: 23525947 Free PMC article. - Expression and Role of the Intermediate-Conductance Calcium-Activated Potassium Channel KCa3.1 in Glioblastoma.
Catacuzzeno L, Fioretti B, Franciolini F. Catacuzzeno L, et al. J Signal Transduct. 2012;2012:421564. doi: 10.1155/2012/421564. Epub 2012 May 17. J Signal Transduct. 2012. PMID: 22675627 Free PMC article. - Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance.
Hatanpaa KJ, Burma S, Zhao D, Habib AA. Hatanpaa KJ, et al. Neoplasia. 2010 Sep;12(9):675-84. doi: 10.1593/neo.10688. Neoplasia. 2010. PMID: 20824044 Free PMC article. Review. - Identification of Paxillin as a Prognostic Factor for Glioblastoma via Integrated Bioinformatics Analysis.
Huang Z, Wang H, Sun D, Liu J. Huang Z, et al. Biomed Res Int. 2022 Jun 23;2022:7171126. doi: 10.1155/2022/7171126. eCollection 2022. Biomed Res Int. 2022. PMID: 35782068 Free PMC article. Retracted. - PTPN6-EGFR Protein Complex: A Novel Target for Colon Cancer Metastasis.
Liu G, Zhang Y, Huang Y, Yuan X, Cao Z, Zhao Z. Liu G, et al. J Oncol. 2022 Feb 11;2022:7391069. doi: 10.1155/2022/7391069. eCollection 2022. J Oncol. 2022. PMID: 35186080 Free PMC article.
References
- Amberger VR, Hensel T, Ogata N, Schwab ME. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998;58:149–158. - PubMed
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and Ink4a/Arf. Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. - PubMed
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines. Adv Immunol. 1994;55:97–179. - PubMed
- Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. - PubMed
- Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, Cervellera MN, Di Cioccio V, Cesta MC, Galliera E, Martinez FO, Di Bitondo R, Troiani G, Sabbatini V, D'Anniballe G, Anacardio R, Cutrin JC, Cavalieri B, Mainiero F, Strippoli R, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA. 2004;101:11791–11796. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS047188-02/NS/NINDS NIH HHS/United States
- NS051255/NS/NINDS NIH HHS/United States
- R01 NS051255-04/NS/NINDS NIH HHS/United States
- R01 NS047188/NS/NINDS NIH HHS/United States
- R01 CA57683/CA/NCI NIH HHS/United States
- R01 CA057683/CA/NCI NIH HHS/United States
- R01 NS041021-08/NS/NINDS NIH HHS/United States
- NS41021/NS/NINDS NIH HHS/United States
- NS047188/NS/NINDS NIH HHS/United States
- R01 NS041021/NS/NINDS NIH HHS/United States
- R01 NS051255/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous