Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61 - PubMed (original) (raw)
Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61
Kazuhiko Kurozumi et al. Mol Ther. 2008 Aug.
Abstract
Oncolytic viral therapy is under evaluation for toxicity and efficacy in clinical trials relating to several different tumors. We report a significant increase in the angiogenic index of oncolytic virus (OV)-treated glioma-matrigel implants (2.83-fold, P < 0.02). In a rat intracranial glioma model, large tumors from OV-treated animals were significantly more angiogenic than the phosphate-buffered saline (PBS)-treated control tumors (OV: 101 +/- 21.6; PBS: 19.8 +/- 10; P = 0.0037). Transcript profiling of OV-treated tumors revealed dysregulation of several transcripts involved in glioma angiogenesis. OV-mediated induction of CYR61 gene expression (8.94-fold, P = 0.001) correlated significantly with the presence of OV in tumor tissue in vivo (R = 0.7, P < 0.001). Further, induction of CYR61 mRNA and protein were confirmed in multiple human cancer cell lines and primary human tumor-derived cells in vitro, and in tumor lysate and cerebrospinal fluid (CSF) in vivo. Finally, we show that treatment of glioma cells with Cilengitide, known to counter CYR61-induced integrin activation, significantly suppressed the proangiogenic effect of OV treatment of gliomas (P < 0.05).
Figures
Figure 1. Treatment of human glioma cells with oncolytic virus (OV) increases their angiogenic potential
(a) Images of individual angioreactors containing HSVQ-treated (top) or phosphate-buffered saline (PBS)-treated LN229 glioma cells at the time of harvesting. Briefly, angioreactors filled with LN229 glioma cells treated with either PBS or HSVQ (n = 9/group) were implanted subcutaneously in athymic nude mice. Fifteen days after implantation, the angioreactors were harvested and analyzed visually for blood vessels that had developed into the tubes. The presence of visually obvious initiation of blood vessels into six of the nine HSVQ-treated angioreactors and into one of the nine PBS-treated angioreactors are indicated by arrow heads. (b) Quantification of hemoglobin (Hb) in angioreactors treated with HSVQ or PBS. The contents of the individual angioreactors were isolated and the amount of Hb in each tube was quantified. Note the significantly higher values of Hb in angioreactors filled with HSVQ-treated LN229 cells as compared to values in angioreactors with PBS-treated LN229 cells (2.58 times higher, P < 0.021). HSV-1, herpes simplex virus 1.
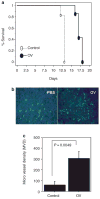
Figure 2. Induction of angiogenesis in oncolytic virus (OV)-treated tumors in a syngeneic rat glioma model
(a) Kaplan–Meier analysis of rats with brain tumors treated with phosphate-buffered saline (PBS) or hrR3. Seven days after intracranial tumor implantation (D74/HveC glioma cells), rats were treated with PBS or hrR3 by direct intratumoral injection. All the rats were monitored closely and killed when they showed signs of morbidity. Note the significant increase in survival of tumor bearing rats treated with hrR3 as compared to PBS-treated rats (P = 0.0006). (b) Fluorescent microscopy images of tumor sections derived from PBS- or OV-treated animals at the time of killing the animals. Rats, with intracranial tumors treated with PBS or hrR3, were killed when they displayed signs of morbidity. Five minutes before being killed, the rats were injected with fluorescein isothiocyanate–conjugated dextran by tail-vein injection. Brain sections from the animals were analyzed by fluorescence microscopy for perfused blood vessels. Note the substantial increase in vascular density in OV-treated tumors as compared to PBS-treated tumors. (c) Quantification of microvessel density (MVD) in tumor sections from rats treated with PBS or hrR3. Note the significant increase in MVD in tumors of OV-treated rats (P = 0.0049).
Figure 3. Correlation between CYR61 and _β_-galactosidase (β-gal), but not between TSP-1 and Ang-2 levels, with oncolytic virus (OV) encoded lacZ
(a–c) Quantitative real-time PCR (QRT-PCR) for OV-encoded (a) CYR61, (b) Ang-2, and (c) TSP-1 gene expression in rat brain tumors. Rats with intracranial brain tumors were pretreated with phosphate-buffered saline (PBS)/cyclophosphamide (CPA) on day 5, and with PBS/OV on day 7 after tumor cell implantation. Seventy-two hours after OV infection, the animals were killed, and the amounts of Ang-2, CYR61, and TSP-1 messenger RNA (mRNA) relative to β-actin were quantified using QRT-PCR (n = 9/group). There was a significant increase in CYR61 mRNA in animals treated with OV + CPA as compared to animals treated with OV alone. (d,e) Scatter plot analysis of the expression of OV encoded (d) β-gal versus CYR61, (e) Ang 2, and (f) TSP-1 rat glioma tumor tissue, both with and without CPA pretreatment. Note the significant positive correlation between CYR61 and β-gal (R = 0.710, P = 0.0009), but not between β-gal and Ang-2 (R = 0.189, P = 0.451) or between β-gal and TSP-1 (R = 0.268, P = 0.283).
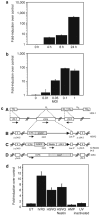
Figure 4. Rapid and dose-dependent induction of CYR61 messenger RNA (mRNA) in rat glioma cells in vitro
(a) D74 cells were infected with hrR3 in vitro, and cell lysates were harvested 0, 4, 8, and 24 hours after infection. After RNA isolation and complementary DNA synthesis, the samples were analyzed for CYR61 expression using quantitative realtime PCR (QRT-PCR). The results were standardized by expressing CYR61 induction as a multiple of actin expression in each sample. Fold change in CYR61 levels relative to uninfected cells reveals, a rapid induction of CYR61 after hrR3 infection of glioma cells. (b) Induction of cellular CYR61 consequent to oncolytic virus (OV) infection displays a dose-response relationship with the number of infectious viral particles used. D74/HveC rat glioma cells were plated and infected with hrR3 at the indicated multiplicity of infection (MOI). Total RNA from cells was harvested 24 hours after infection and analyzed for CYR61 mRNA expression relative to actin. Note that induction of CYR61 increases with increase in infectious particles. (c) Schematic of the various herpes simplex virus-1 (HSV-1)-derived OVs used in this study. (A) HSV-1 genome structure showing the UL (unique long sequences) and US (unique small sequences). Enlargements of the UL region depict the γ34.5 and ICP6 domains. (B) HSVQ is an HSV-1-derived virus with deletions of both γ34.5 genes and interruption of ICP6 by an insertion of enhanced green fluorescent protein (EGFP). This renders the virus unable to replicate in nondividing cells. (C) rQNestin34.5 is an HSVQ-derived virus in which one copy of the γ34.5 gene is reinserted into the UL39 locus under a nestin promoter, making the virus more potent in nestin-positive glioma-initiating cells. (D) hrR3 is an HSV-1-derived OV with an in-frame insertion of LacZ within its ICP6 locus, causing disruption of the viral ribonucleotide reductase gene. (d) In vitro, D74 rat glioma cells were infected with replication-incompetent amplicon, ultraviolet (UV)-inactivated hrR3, or the indicated OV. Total cellular RNA was harvested 24 hours after infection and analyzed for expression of cellular CYR61 relative to actin, as described in Materials and Methods. Note the significant induction of CYR61 mRNA expression in each of the three OVs relative to the uninfected sample. No significant induction was observed after replication-deficient amplicon infection or UV-inactive hrR3 infection of cells.
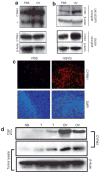
Figure 5. Induction of CYR61 protein in vitro and in vivo consequent to oncolytic virus (OV) infection
(a) U343 human glioma cells were infected with hrR3 at a multiplicity of infection of 0.05. The cell lysate and extracellular matrix were harvested and analyzed for CYR61 expression using western blot. Note the significant induction of CYR61 in U343 cells infected with hrR3 relative to uninfected control cells. Asterisk indicates a proteolytic fragment of secreted CYR61 in U343 cells. Proteolytic processing of CYR61 has been described earlier. (b) Intracranial (human U87ΔEGFR glioma cells) and subcutaneous (LN229) tumors generated in athymic nude mice were treated with HSVQ or phosphate-buffered saline (PBS). The animals were killed 24 hours after treatment, and the harvested tumor lysate was analyzed for CYR61 expression using western blot. Note the substantial induction of CYR61 protein in tumors infected with HSVQ. (c) Representative immunofluorescent tumor sections from U87ΔEGFR tumors treated with OV/PBS in vivo. Seven days after tumor cell implantation, athymic nude mice were treated with PBS or hrR3, and killed 3 days after treatment. Sections from the tumor-containing brains were analyzed by immunofluorescence for CYR61 protein expression (red, top). Nuclear [4′,6-diamidino-2-phenylindole (DAPI)] staining (blue, bottom) shows the tumors containing highly nucleated area in the sections. (d) Rats with intracranial tumors (D74/HveC glioma cells) were treated with hrR3 7 days after tumor implantation. Twenty-four hours after OV treatment, cerebrospinal fluid (CSF) and tumors were harvested from the rats, as described in Materials and Methods. The harvested CSF and tumor lysate were analyzed for CYR61 and β-actin, using western blot. Note the increase in CYR61 protein in the CSF of rats with tumors treated with OV relative to tumor-bearing control rats treated with PBS.
Figure 6. Impact of OV-induced CYR61 on glioma biology and oncolysis
(a) Images of individual angioreactors containing HSVQ-treated (top) and phosphate-buffered saline (PBS)-treated LN229 glioma cells at the time of harvesting. Briefly, angioreactors filled with LN229 glioma cells treated with either PBS or HSVQ (n = 9/group) in the presence or absence of Cilengitide (30 ng/angioreactor) were implanted subcutaneously in athymic nude mice. Fifteen days after implantation the angioreactors were harvested and analyzed visually for blood vessels that had developed into the tubes. The presence of visually obvious initiation of blood vessels into six of the nine HSVQ-treated angioreactors and in three of the nine PBS-treated angioreactors are indicated by arrow heads. (b) Quantification of hemoglobin (Hb) in angioreactors: The contents of the individual angioreactors were isolated and the amount of Hb in each tube was quantified. Note the significant reduction in Hb content of angioreactors treated with HSVQ in the presence of Cilengitide (P ≤ 0.036).
Similar articles
- The integrin inhibitor cilengitide enhances the anti-glioma efficacy of vasculostatin-expressing oncolytic virus.
Fujii K, Kurozumi K, Ichikawa T, Onishi M, Shimazu Y, Ishida J, Chiocca EA, Kaur B, Date I. Fujii K, et al. Cancer Gene Ther. 2013 Aug;20(8):437-44. doi: 10.1038/cgt.2013.38. Epub 2013 Jul 5. Cancer Gene Ther. 2013. PMID: 23827879 Free PMC article. - Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat.
Li Z, Yan G, Diao Q, Yu F, Li X, Sheng X, Liu Y, Dai Y, Zhou H, Zhen X, Hu Y, Péault B, Ding L, Sun H, Li H. Li Z, et al. Stem Cell Res Ther. 2019 Jun 18;10(1):179. doi: 10.1186/s13287-019-1272-3. Stem Cell Res Ther. 2019. PMID: 31215503 Free PMC article. - Active immunotherapy: oncolytic virus therapy using HSV-1.
Todo T. Todo T. Adv Exp Med Biol. 2012;746:178-86. doi: 10.1007/978-1-4614-3146-6_14. Adv Exp Med Biol. 2012. PMID: 22639168 Review. - Oncolytic HSV-1 for the treatment of brain tumours.
Markert JM, Parker JN, Buchsbaum DJ, Grizzle WE, Gillespie GY, Whitley RJ. Markert JM, et al. Herpes. 2006 Nov;13(3):66-71. Herpes. 2006. PMID: 17147910 Review.
Cited by
- Immunosuppressive cells in oncolytic virotherapy for glioma: challenges and solutions.
Liu J, Piranlioglu R, Ye F, Shu K, Lei T, Nakashima H. Liu J, et al. Front Cell Infect Microbiol. 2023 May 10;13:1141034. doi: 10.3389/fcimb.2023.1141034. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37234776 Free PMC article. Review. - Cross-sectional and longitudinal associations of serum Cysteine-rich 61 with severity and prognosis among community-acquired pneumonia patients in China.
Yao MX, Cheng JY, Liu Y, Sun J, Hua DX, He QY, Liu HY, Fu L, Zhao H. Yao MX, et al. Front Med (Lausanne). 2022 Aug 11;9:939002. doi: 10.3389/fmed.2022.939002. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36035395 Free PMC article. - G-quadruplexes Stabilization Upregulates CCN1 and Accelerates Aging in Cultured Cerebral Endothelial Cells.
Noh B, Blasco-Conesa MP, Lai YJ, Ganesh BP, Urayama A, Moreno-Gonzalez I, Marrelli SP, McCullough LD, Moruno-Manchon JF. Noh B, et al. Front Aging. 2022 Jan 12;2:797562. doi: 10.3389/fragi.2021.797562. eCollection 2021. Front Aging. 2022. PMID: 35822045 Free PMC article. - Oncolytic Viruses: Immunotherapy Drugs for Gastrointestinal Malignant Tumors.
Li Q, Oduro PK, Guo R, Li R, Leng L, Kong X, Wang Q, Yang L. Li Q, et al. Front Cell Infect Microbiol. 2022 Jun 3;12:921534. doi: 10.3389/fcimb.2022.921534. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35719333 Free PMC article. Review. - The CREB and AP-1-Dependent Cell Communication Network Factor 1 Regulates Porcine Epidemic Diarrhea Virus-Induced Cell Apoptosis Inhibiting Virus Replication Through the p53 Pathway.
Zhou H, Zhang Y, Wang J, Yan Y, Liu Y, Shi X, Zhang Q, Xu X. Zhou H, et al. Front Microbiol. 2022 Mar 28;13:831852. doi: 10.3389/fmicb.2022.831852. eCollection 2022. Front Microbiol. 2022. PMID: 35418961 Free PMC article.
References
- Brat DJ, Castellano-Sanchez A, Kaur B, Van Meir EG. Genetic and biologic progression in astrocytomas and their relation to angiogenic dysregulation. Adv Anat Pathol. 2002;9:24–36. - PubMed
- Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. - PubMed
- Hardcastle J, Kurozumi K, Chiocca EA, Kaur B. Oncolytic viruses driven by tumor-specific promoters. Curr Cancer Drug Targets. 2007;7:181–189. - PubMed
- Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. - PubMed
- Fukuhara H, Todo T. Oncolytic herpes simplex virus type 1 and host immune responses. Curr Cancer Drug Targets. 2007;7:149–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA069246-110004/CA/NCI NIH HHS/United States
- 1K01NS059575/NS/NINDS NIH HHS/United States
- K01 NS059575/NS/NINDS NIH HHS/United States
- R21 NS056203-01A2/NS/NINDS NIH HHS/United States
- R01 NS064607/NS/NINDS NIH HHS/United States
- K01 NS059575-01A1/NS/NINDS NIH HHS/United States
- P01 CA069246/CA/NCI NIH HHS/United States
- R21 NS056203/NS/NINDS NIH HHS/United States
- R01NS064607/NS/NINDS NIH HHS/United States
- R01 NS064607-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources