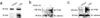Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke - PubMed (original) (raw)
doi: 10.1038/nm1787. Epub 2008 Jun 22.
Linda Fredriksson, Melissa Geyer, Erika Folestad, Jacqueline Cale, Johanna Andrae, Yamei Gao, Kristian Pietras, Kris Mann, Manuel Yepes, Dudley K Strickland, Christer Betsholtz, Ulf Eriksson, Daniel A Lawrence
Affiliations
- PMID: 18568034
- PMCID: PMC2811427
- DOI: 10.1038/nm1787
Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke
Enming J Su et al. Nat Med. 2008 Jul.
Abstract
Thrombolytic treatment of ischemic stroke with tissue plasminogen activator (tPA) is markedly limited owing to concerns about hemorrhagic complications and the requirement that tPA be administered within 3 h of symptoms. Here we report that tPA activation of latent platelet-derived growth factor-CC (PDGF-CC) may explain these limitations. Intraventricular injection of tPA or active PDGF-CC, in the absence of ischemia, leads to significant increases in cerebrovascular permeability. In contrast, co-injection of neutralizing antibodies to PDGF-CC with tPA blocks this increased permeability, indicating that PDGF-CC is a downstream substrate of tPA within the neurovascular unit. These effects are mediated through activation of PDGF-alpha receptors (PDGFR-alpha) on perivascular astrocytes, and treatment of mice with the PDGFR-alpha antagonist imatinib after ischemic stroke reduces both cerebrovascular permeability and hemorrhagic complications associated with late administration of thrombolytic tPA. These data demonstrate that PDGF signaling regulates blood-brain barrier permeability and suggest potential new strategies for stroke treatment.
Figures
Figure 1
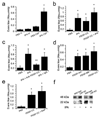
Active PDGF-CC mediates tPA-induced cerebrovascular permeability. (a) Comparison of EB extravasation 1 h following either intravenous injection (IV) with 10 mg/kg of tPA as a bolus (~250 µg/mouse) or intraventricular injection (CSF) with 585 ng of tPA. (b) EB extravasation 1 h after intraventricular injection of PBS (PBS), tPA (tPA), active PDGF-CC (PDGF-CC), or active PDGF-CC together with tPA (PDGF-CC+tPA). (c) EB extravasation 1 h after intraventricular injection of either PBS (PBS), active tPA (tPA), tPA with blocking antibodies to PDGF-CC (tPA+anti-PDGF-CC), or tPA together with control IgG (tPA+IgG). (d) EB extravasation 1 h after intraventricular injection of PBS (PBS), PDGF-AA (PDGF-AA), PDGF-BB (PDGF-BB), or active PDGF-CC (PDGF-CC). (e) EB extravasation 1 h after intraventricular injection with either PBS (PBS), active PDGF-CC (PDGF-CC), or active PDGF-CC together with the LRP antagonist RAP (PDGF-CC+RAP). For all injections into the CSF, 3 µl of 3 µM protein was used except for antibodies which were 0.4 mg/ml. Each group n = 8–10 and errors represent S.E.M. Single asterisks indicate p < 0.01 vs. PBS, and ** indicates p < 0.05 vs the IgG control. (f) PDGF-CC cleavage by tPA is impaired in _Lrp_−/− cells (LRP KO). Serum free medium from _Lrp_−/− MEFs and wild-type cells demonstrates that both cell lines express the 48 kDa full length PDGF-C, while the addition of exogenous tPA to the cells only generates the 22 kDa PDGF-C species in the presence of the wild type cells but not in _Lrp_−/− cells.
Figure 2
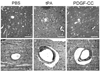
TPA and PDGF-CC induce similar morphological changes in brain vasculature. (a–c) Light microscopy images of cerebral sections prepared for EM analysis but stained with toluene blue. (d–f) High magnification micrographs from electron microscopy of cerebral arterioles. Brains were harvested 1 h after intraventricular injection of either PBS (a and d), tPA (b and e) or PDGF-CC (c and f). Scale bar is 50 µM in a–c and 2 µM in d–f.
Figure 3
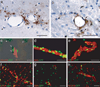
PDGF-CC, tPA and the PDGFR-α are expressed in the NVU. (a) Sections from normal mouse brains stained with antibodies to PDGF-CC show PDGF-CC staining in patches associated with arterioles (arrows), but not with capillaries (arrowheads). (b) Sections from normal mouse brains stained with antibodies to tPA show mainly perivascular tPA staining in association with an arteriole (arrows). (c–h) Micrographs showing PDGFR-α expression in mouse brain using a GFP reporter. (c) Sections of double heterozygous _Pdgfr_α+/GFP/Pdgfc+/lacZ mouse brains stained with the PDGF-C reporter Xgal and antibodies to the endothelial cell marker platelet/endothelial cell adhesion molecule-1 (PECAM red). The arrows indicate vessel associated PDGF-C expression and arrowheads indicate non-vessel associated expression. GFP and PECAM staining was visualized using fluorescence, whereas Xgal staining was viewed in bright field. (d,e) Whole mount immunofluorescence staining of GFP-positive vessel fragments stained for SMA (red) (d) confirmed that the GFP-positive vessel fragments are arterioles. The GFP-positive nuclei are mainly localized outside of the SMA-positive cells. In contrast, co-staining with GFAP (red) (e), an astrocyte marker, suggests that the GFP-positive cells are astrocytes. (f–h) Immunofluorescence staining of brain sections from _Pdgfr_α+/GFP mice stained with markers for astrocytes, GFAP (red) (f), vascular smooth muscle cells, SMA (red) (g), and endothelial cells, PECAM (red) (h). Similar to the isolated vessel fragments, co-localization of GFP expression with GFAP was abundant in the stained brain sections and produced a yellow color (g). Scale bar is 50 µm in a–b, d–h, and 20 µm in c.
Figure 4
PDGF-CC is expressed by astrocytes in culture. (a–c) Immunoblot analysis of PDGFCC in astrocytes. (a) A 48 kDa band corresponding to full length PDGF-C is seen in control cell media whereas addition of tPA (tPA) to the cells prior to collection of the medium induces release of the active 22 kDa PDGF-C species. PDGFR-α expressed by astrocytes in culture is stimulated by addition of active PDGF-CC (b) or tPA (c) to the cells. Receptor tyrosine phosphorylation is determined by immunoblotting (IB) of cell lysates for phosphotyrosine. Immunoblotting calnexin is also shown as a loading control.
Figure 5
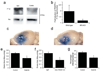
Blocking PDGFR-α activation reduces cerebrovascular permeability and stroke volume after MCAO. (a) PDGFR-α activation following MCAO in wild-type mice 6 h after MCAO. Brains were divided into ipsilateral and contralateral hemispheres, detergent-solubilized and the lysates immunoprecipitated with PDGFR-α-specific antibodies followed by immunoblotting with phosphotyrosine-specific antibodies. As a loading control, the precipitated protein was visualized with PDGFR-α-specific antibodies. (b) Quantitation of PDGFR-α activation following MCAO in wild-type or tPA KO mice (n = 2 in each group and errors represent S.E.M). (c,d) Representative images of the ipsilateral hemisphere 24 h after MCAO from control (c) and Imatinib-treated mice (d) injected with EB 1 h before euthanasia. (e) Quantification of the EB extravasation 24 h after MCAO (n = 11 for each group and errors represent S.E.M.). (f) Quantification of the EB extravasation 24 h after MCAO in mice treated with intraventricular PBS, preimmune IgG or PDGF-CC-specific antibodies followed immediately by MCAO (n = 10 for each group and errors represent S.E.M). (g) Quantification of infarct size 72 h after MCAO in mice treated with either Imatinib or vehicle (n = 9 for control group and n = 11 for Imatinib group and errors represent S.E.M.). For all Imatinib studies mice were treated by gavage with either vehicle or 200 mg/kg Imatinib 1 h and 8 h after MCAO and twice daily for the duration of the experiment. In panels e–g the asterisks indicate p < 0.05 vs. control animals. Scale bar is 2 mm in c and d.
Figure 6
Blocking the PDGF-CC/PDGFR-α pathway reduces intracerebral hemorrhage after MCAO. (a,b) Representative images of the intracerebral hemorrhage in the ipsilateral hemisphere 24 h after MCAO of animals treated with tPA 5 h after MCAO. Mice were treated by gavage with either vehicle (a) or 200 mg/kg Imatinib (b) 1 h and 8 h after MCAO. (c) Quantification of hemoglobin content in the ischemic hemisphere 24 h after MCAO (n = 10 for control group and n = 12 for Imatinib group and errors represent S.E.M). The asterisk indicate p < 0.05 vs. control animals. Scale bar is 2 mm in a and b.
Comment in
- Imatinib buys time for brain after stroke.
Rieckmann P. Rieckmann P. Nat Med. 2008 Jul;14(7):712-3. doi: 10.1038/nm0708-712. Nat Med. 2008. PMID: 18607366 No abstract available.
Similar articles
- Microglial-mediated PDGF-CC activation increases cerebrovascular permeability during ischemic stroke.
Su EJ, Cao C, Fredriksson L, Nilsson I, Stefanitsch C, Stevenson TK, Zhao J, Ragsdale M, Sun YY, Yepes M, Kuan CY, Eriksson U, Strickland DK, Lawrence DA, Zhang L. Su EJ, et al. Acta Neuropathol. 2017 Oct;134(4):585-604. doi: 10.1007/s00401-017-1749-z. Epub 2017 Jul 19. Acta Neuropathol. 2017. PMID: 28725968 Free PMC article. - Remote ischemic conditioning attenuates blood-brain barrier disruption after recombinant tissue plasminogen activator treatment via reducing PDGF-CC.
He Q, Ma Y, Fang C, Deng Z, Wang F, Qu Y, Yin M, Zhao R, Zhang D, Guo F, Yang Y, Chang J, Guo ZN. He Q, et al. Pharmacol Res. 2023 Jan;187:106641. doi: 10.1016/j.phrs.2022.106641. Epub 2022 Dec 29. Pharmacol Res. 2023. PMID: 36587812 - Platelet derived growth factor-CC isoform is associated with hemorrhagic transformation in ischemic stroke patients treated with tissue plasminogen activator.
Rodríguez-González R, Blanco M, Rodríguez-Yáñez M, Moldes O, Castillo J, Sobrino T. Rodríguez-González R, et al. Atherosclerosis. 2013 Jan;226(1):165-71. doi: 10.1016/j.atherosclerosis.2012.10.072. Epub 2012 Nov 17. Atherosclerosis. 2013. PMID: 23218119 - Tissue plasminogen activator-mediated PDGF signaling and neurovascular coupling in stroke.
Su EJ, Fredriksson L, Schielke GP, Eriksson U, Lawrence DA. Su EJ, et al. J Thromb Haemost. 2009 Jul;7 Suppl 1(Suppl 1):155-8. doi: 10.1111/j.1538-7836.2009.03402.x. J Thromb Haemost. 2009. PMID: 19630790 Free PMC article. Review. - Pharmacological targeting of the PDGF-CC signaling pathway for blood-brain barrier restoration in neurological disorders.
Lewandowski SA, Fredriksson L, Lawrence DA, Eriksson U. Lewandowski SA, et al. Pharmacol Ther. 2016 Nov;167:108-119. doi: 10.1016/j.pharmthera.2016.07.016. Epub 2016 Aug 12. Pharmacol Ther. 2016. PMID: 27524729 Free PMC article. Review.
Cited by
- D1 receptor-mediated endogenous tPA upregulation contributes to blood-brain barrier injury after acute ischaemic stroke.
Wang Y, Wang X, Zhang X, Chen S, Sun Y, Liu W, Jin X, Zheng G. Wang Y, et al. J Cell Mol Med. 2020 Aug;24(16):9255-9266. doi: 10.1111/jcmm.15570. Epub 2020 Jul 6. J Cell Mol Med. 2020. PMID: 32627929 Free PMC article. - Deficiency in Neuroserpin Exacerbates CoCl2 Induced Hypoxic Injury in the Zebrafish Model by Increased Oxidative Stress.
Han S, Zhang D, Dong Q, Wang X, Wang L. Han S, et al. Front Pharmacol. 2021 Mar 2;12:632662. doi: 10.3389/fphar.2021.632662. eCollection 2021. Front Pharmacol. 2021. PMID: 33737878 Free PMC article. - Osteoblasts Have a Neural Origin in Heterotopic Ossification.
Lazard ZW, Olmsted-Davis EA, Salisbury EA, Gugala Z, Sonnet C, Davis EL, Beal E 2nd, Ubogu EE, Davis AR. Lazard ZW, et al. Clin Orthop Relat Res. 2015 Sep;473(9):2790-806. doi: 10.1007/s11999-015-4323-9. Clin Orthop Relat Res. 2015. PMID: 25944403 Free PMC article. - Imatinib attenuates cerebrovascular injury and phenotypic transformation after intracerebral hemorrhage in rats.
Pearce WJ, Doan C, Carreon D, Kim D, Durrant LM, Manaenko A, McCoy L, Obenaus A, Zhang JH, Tang J. Pearce WJ, et al. Am J Physiol Regul Integr Comp Physiol. 2016 Dec 1;311(6):R1093-R1104. doi: 10.1152/ajpregu.00240.2016. Epub 2016 Oct 5. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27707720 Free PMC article. - The tissue-type plasminogen activator-plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: evidence from mice and humans.
Sashindranath M, Sales E, Daglas M, Freeman R, Samson AL, Cops EJ, Beckham S, Galle A, McLean C, Morganti-Kossmann C, Rosenfeld JV, Madani R, Vassalli JD, Su EJ, Lawrence DA, Medcalf RL. Sashindranath M, et al. Brain. 2012 Nov;135(Pt 11):3251-64. doi: 10.1093/brain/aws178. Epub 2012 Jul 20. Brain. 2012. PMID: 22822039 Free PMC article.
References
- Thom T, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. - PubMed
- Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int. Rev. Cytol. 2002;221:93–148. - PubMed
- Marler JR, Goldstein LB. Medicine. Stroke--tPA and the clinic. Science. 2003;301:1677. - PubMed
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL057346/HL/NHLBI NIH HHS/United States
- R01 HL050784/HL/NHLBI NIH HHS/United States
- P01 HL054710/HL/NHLBI NIH HHS/United States
- HL55374/HL/NHLBI NIH HHS/United States
- R01 NS062073/NS/NINDS NIH HHS/United States
- R01 HL055374-10/HL/NHLBI NIH HHS/United States
- P01 HL054710-120006/HL/NHLBI NIH HHS/United States
- R01 HL055374-09/HL/NHLBI NIH HHS/United States
- R01 NS049478-04/NS/NINDS NIH HHS/United States
- R01 HL055747-10/HL/NHLBI NIH HHS/United States
- R01 HL055374-11A1/HL/NHLBI NIH HHS/United States
- R01 NS049478/NS/NINDS NIH HHS/United States
- R01 NS079331/NS/NINDS NIH HHS/United States
- NS49478/NS/NINDS NIH HHS/United States
- P01 HL054710-11A10006/HL/NHLBI NIH HHS/United States
- R01 HL055747/HL/NHLBI NIH HHS/United States
- HL55747/HL/NHLBI NIH HHS/United States
- R01 HL055374-12/HL/NHLBI NIH HHS/United States
- R01 HL050784-09/HL/NHLBI NIH HHS/United States
- R01 HL055374/HL/NHLBI NIH HHS/United States
- P01 HL054710-11A1/HL/NHLBI NIH HHS/United States
- R01 HL055747-11/HL/NHLBI NIH HHS/United States
- HL54710/HL/NHLBI NIH HHS/United States
- HL57346/HL/NHLBI NIH HHS/United States
- P01 HL054710-12/HL/NHLBI NIH HHS/United States
- R01 NS091201/NS/NINDS NIH HHS/United States
- P01 HL057346-11A18577/HL/NHLBI NIH HHS/United States
- HL50784/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous