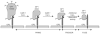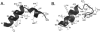Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme - PubMed (original) (raw)
Review
Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme
Judith M White et al. Crit Rev Biochem Mol Biol. 2008 May-Jun.
Erratum in
- Crit Rev Biochem Mol Biol. 2008 Jul-Aug;43(4):287-8
Abstract
Recent work has identified three distinct classes of viral membrane fusion proteins based on structural criteria. In addition, there are at least four distinct mechanisms by which viral fusion proteins can be triggered to undergo fusion-inducing conformational changes. Viral fusion proteins also contain different types of fusion peptides and vary in their reliance on accessory proteins. These differing features combine to yield a rich diversity of fusion proteins. Yet despite this staggering diversity, all characterized viral fusion proteins convert from a fusion-competent state (dimers or trimers, depending on the class) to a membrane-embedded homotrimeric prehairpin, and then to a trimer-of-hairpins that brings the fusion peptide, attached to the target membrane, and the transmembrane domain, attached to the viral membrane, into close proximity thereby facilitating the union of viral and target membranes. During these conformational conversions, the fusion proteins induce membranes to progress through stages of close apposition, hemifusion, and then the formation of small, and finally large, fusion pores. Clearly, highly divergent proteins have converged on the same overall strategy to mediate fusion, an essential step in the life cycle of every enveloped virus.
Figures
FIG. 1
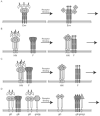
The common trimer-of-hairpins pathway of membrane fusion. (A) The model depicts a Class I fusion protein, but related structures (e.g., prehairpins and trimers-of-hairpins) form for Class II and III proteins, which also promote membrane merger through stages of close apposition (iv), hemifusion (v), small fusion pores (not shown), and large fusion pores (vi). See Table 2 and text for comparisons among the different classes of viral fusion proteins. The depicted Class I fusion protein is one that does not require any other viral surface proteins for fusion (e.g., influenza HA or a retroviral Env); it contains both a receptor binding subunit (labeled rb in image i) and a fusion subunit (labeled f in images i to iii). The target and viral membranes are, respectively, at the top and bottom of the images. The receptor binding subunit (rb) is not shown beyond image i as its location at the later stages is not known; in all cases studied, however, the rb subunit of this type of class I fusion protein must move out of the way, thus unclamping the fusion subunit in the metastable fusion competent state and allowing fusion to proceed. For Class I fusion proteins six helix bundles (6HBs) are seen in their bundle (v) and trimer-of-hairpins (vi) forms; the length and position of the 6HB varies for different proteins. The starting (i) and final (vi) images represent structures that are known for several viral fusion proteins; high level structural information is currently lacking on the intermediates. (B) The key features of a class I fusion protein from N- to C-terminus: a fusion peptide (FP) at or near the N-terminus, an N-heptad repeat (N-HR aka HR1 or HRA), a C-heptad repeat (C-HR aka HR2 or HRB), a transmembrane domain (TMD), and a cytoplasmic tail (squiggle). Linkers of variable lengths are indicated as straight lines. (The // between the N- and C-heptad repeats indicates that the length of these linkers varies considerably). Peptide analogs of the N-HR and C-HR helices can inhibit fusion and infection.
FIG. 2
Diversity of viral fusion proteins. The major differences among viral fusion proteins are their structural class (left) and mode of fusion triggering (right). Representative fusion proteins of different classes that employ different fusion triggers are: (A) influenza HA, (B) paramyxovirus F and most retroviral Env proteins, (C) _α_-retroviral Env proteins, (D) Ebola GP, (E) the E and E1 proteins, respectively, of TBEV and SFV, (F) VSV G, and (G) HSV gB. Some herpesviruses require low pH (likely in addition to receptor binding) for fusion in certain cell types (see text). Additional differences among viral fusion proteins include the locations and types of their fusion peptides and whether they require additional viral surface proteins (e.g., separate receptor binding proteins) for fusion.
FIG. 3
Receptor activation of viral fusion proteins. Many viral fusion proteins are activated by host cell receptors, but there are many variations on how this happens (see text). Selected examples are cartooned: (A) Moloney MLV Env, (B) a paramyxovirus F according to the “association” model, (C) a paramyxovirus F according to the “dissociation” model. (D) A highly speculative model for HSV. Specific protein names are indicated under the respective proteins. The models show only the first step of fusion activation, formation of the prehairpin intermediate; subsequent steps (right arrow) lead in all cases to formation of a trimer-of-hairpins. Designations are: R, receptor; rb, receptor binding subunit; f, fusion subunit. The up arrows associated with the known fusion subunits (f) in the right hand images of all panels indicate the exposed fusion peptides, which insert into the target membrane. In (A, right panel) the receptor binding subunit is shown loosely attached (dotted line) to the fusion subunit (f). In a subsequent step, the rb dissociates (see text and Figure 4). In (D) gB is shown in a possible prehairpin conformation as is gH/gL (large upward arrow) consistent with the participation of both gB and gH/gL in fusion, but their exact roles at all stages of fusion remain to be clarified. Receptors are not shown in the right hand images, as it is not yet clear whether they remain bound at this and/or later stages.
FIG. 4
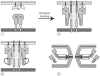
Model for receptor activation of the Moloney MLV Env protein. The figure is modeled after Figure 7D in (Wallin et al., 2004), but modified based on new information (see text). In the first image (i) the fusion subunit is depicted as being largely hidden. Following receptor (R) binding, a conformational change exposes the CXXC motif in the receptor binding subunit (rb) allowing the fusion subunit to form the prehairpin intermediate (image ii). A subsequent internal thiol disulfide exchange reaction (image iii) leads to dissociation of the receptor binding subunit, allowing the fusion subunit to fold back into a trimer-of-hairpins. At some point, the receptor binding subunit (rb) likely disengages from its receptor, but it is not yet clear when this happens. Similar models may apply to some, but not all, other retroviral Env proteins (see text).
FIG. 5
Model for priming and triggering of the Ebola virus glycoprotein. The figure is modified from Figure 4A in (Schornberg et al., 2006). GP1 is the receptor binding subunit and GP2 is the fusion subunit. Cat B and Cat L denote the endosomal cathepsins (B and L, respectively). 19 kD GP1* denotes the post fusion form of GP1. Prime denotes the cleavage of GP1 to the 19kD form. Trigger denotes the unclamping of GP2, which leads to fusion (Fuse). For clarity only one monomer of the GP trimer is shown.
FIG. 6
Structures of Class I, II, and III fusion proteins in their pre- and post-fusion forms. The crystal structures of the Class I fusion proteins, Influenza virus HA2 (A) and Paramyxovirus F (B), a Class II fusion protein, TBEV E (C), and a Class III fusion protein, VSV G (D), are shown. The pre-fusion states (i and ii) are on the left and the post-fusion states (iii and iv) are on the right with functional domains identified by color. Fusion peptides are in red, with the domains containing them in dark blue. C-terminal domains, which connect to the virus membrane, are in purple. C-terminal linkers, transmembrane domains, and fusion peptides not visible in the structure are represented by dashed purple lines, purple triangles, and red triangles, respectively. Regions important for the movement of the fusion peptide toward the target membrane are displayed in orange, and regions important for C-terminal inversion, that bring the fusion peptide and transmembrane domains together, are shown in green (in Ai-iii, Bi-iii, Ci-ii, and Di-iii). In the post-fusion trimer structures (iv), the orange and green are replaced by dark blue or purple domain coloring, respectively, to illustrate the similarities among all of the post-fusion forms. Other domains are represented in cyan, yellow, and blue-gray. PDB accession numbers for the structures are: 2HMG (Ai and ii); 1QU1 (Aiii and iv); 2B9B (Bi and ii); 1ZTM (Biii and iv); 1SVB (Ci); 1URZ (Cii and iii); 2J6J (Di and ii); 2CMZ (Diii and iv).
FIG. 7
Structures of the HA and HIV fusion peptides in DPC micelles. (A) The HA fusion peptide, residues 1-20 of HA2, sits in the membrane as a kinked structure with E11, N12, and E15 at the membrane interface and the majority of the hydrophobic residues on the interior of the kink (PDB entry 1IBN). F9, I10, and W14 are on the interior at the apex of the kink where they stabilize the bent structure (Lai and Tamm, 2007). The glycine ridge, formed by residues G1, G4, and G8, is visible along the N-terminal helix. (B) The HIV fusion peptide exists as an extended helix from residues I4 to A15 in DPC micelles (PDB entry 2PJV). Both the N- and C-terminal portions of the protein are disordered in the structure. ATR-FTIR studies have shown that the HIV fusion peptide inserts into bilayers at an angle (Martin and Ruysschaert, 2000), but its exact orientation and depth of penetration await further study.
Similar articles
- A Virion-Based Assay for Glycoprotein Thermostability Reveals Key Determinants of Filovirus Entry and Its Inhibition.
Bortz RH 3rd, Wong AC, Grodus MG, Recht HS, Pulanco MC, Lasso G, Anthony SJ, Mittler E, Jangra RK, Chandran K. Bortz RH 3rd, et al. J Virol. 2020 Aug 31;94(18):e00336-20. doi: 10.1128/JVI.00336-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32611759 Free PMC article. - Influenza Hemifusion Phenotype Depends on Membrane Context: Differences in Cell-Cell and Virus-Cell Fusion.
Zawada KE, Okamoto K, Kasson PM. Zawada KE, et al. J Mol Biol. 2018 Mar 2;430(5):594-601. doi: 10.1016/j.jmb.2018.01.006. Epub 2018 Feb 2. J Mol Biol. 2018. PMID: 29355500 Free PMC article. - Viral Membrane Fusion and the Transmembrane Domain.
Barrett CT, Dutch RE. Barrett CT, et al. Viruses. 2020 Jun 27;12(7):693. doi: 10.3390/v12070693. Viruses. 2020. PMID: 32604992 Free PMC article. Review. - Reovirus FAST proteins: virus-encoded cellular fusogens.
Ciechonska M, Duncan R. Ciechonska M, et al. Trends Microbiol. 2014 Dec;22(12):715-24. doi: 10.1016/j.tim.2014.08.005. Epub 2014 Sep 19. Trends Microbiol. 2014. PMID: 25245455 Review.
Cited by
- Filovirus entry: a novelty in the viral fusion world.
Hunt CL, Lennemann NJ, Maury W. Hunt CL, et al. Viruses. 2012 Feb;4(2):258-75. doi: 10.3390/v4020258. Epub 2012 Feb 7. Viruses. 2012. PMID: 22470835 Free PMC article. Review. - Identification of a specific region in the e1 fusion protein involved in zinc inhibition of semliki forest virus fusion.
Liu CY, Kielian M. Liu CY, et al. J Virol. 2012 Apr;86(7):3588-94. doi: 10.1128/JVI.07115-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258261 Free PMC article. - Multiple Strategies Reveal a Bidentate Interaction between the Nipah Virus Attachment and Fusion Glycoproteins.
Stone JA, Vemulapati BM, Bradel-Tretheway B, Aguilar HC. Stone JA, et al. J Virol. 2016 Nov 14;90(23):10762-10773. doi: 10.1128/JVI.01469-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27654290 Free PMC article. - Novel Functions of Hendra Virus G N-Glycans and Comparisons to Nipah Virus.
Bradel-Tretheway BG, Liu Q, Stone JA, McInally S, Aguilar HC. Bradel-Tretheway BG, et al. J Virol. 2015 Jul;89(14):7235-47. doi: 10.1128/JVI.00773-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948743 Free PMC article. - Dynamic changes during acid-induced activation of influenza hemagglutinin.
Garcia NK, Guttman M, Ebner JL, Lee KK. Garcia NK, et al. Structure. 2015 Apr 7;23(4):665-76. doi: 10.1016/j.str.2015.02.006. Epub 2015 Mar 12. Structure. 2015. PMID: 25773144 Free PMC article.
References
- Aguilar HC, Matreyek KA, Filone CM, Hashimi ST, Levroney EL, Negrete OA, Bertolotti-Ciarlet A, Choi DY, McHardy I, Fulcher JA, Su SV, Wolf MC, Kohatsu L, Baum LG, Lee B. N-Glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J Virol. 2006;80:4878–4889. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI22470/AI/NIAID NIH HHS/United States
- R21AI055925/AI/NIAID NIH HHS/United States
- R01 AI022470/AI/NIAID NIH HHS/United States
- R21 AI055925/AI/NIAID NIH HHS/United States
- U54 AI57168/AI/NIAID NIH HHS/United States
- T32 AI007046/AI/NIAID NIH HHS/United States
- U54 AI057168/AI/NIAID NIH HHS/United States
- T32 AI055432/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases