A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus - PubMed (original) (raw)
. 2008 Jul 18;29(1):150-64.
doi: 10.1016/j.immuni.2008.05.012.
Charles Quinn, Jing Shen, Pinakeen Patel, Casey Glaser, Nicole Baldwin, Dorothee Stichweh, Derek Blankenship, Lei Li, Indira Munagala, Lynda Bennett, Florence Allantaz, Asuncion Mejias, Monica Ardura, Ellen Kaizer, Laurence Monnet, Windy Allman, Henry Randall, Diane Johnson, Aimee Lanier, Marilynn Punaro, Knut M Wittkowski, Perrin White, Joseph Fay, Goran Klintmalm, Octavio Ramilo, A Karolina Palucka, Jacques Banchereau, Virginia Pascual
Affiliations
- PMID: 18631455
- PMCID: PMC2727981
- DOI: 10.1016/j.immuni.2008.05.012
A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus
Damien Chaussabel et al. Immunity. 2008.
Abstract
The analysis of patient blood transcriptional profiles offers a means to investigate the immunological mechanisms relevant to human diseases on a genome-wide scale. In addition, such studies provide a basis for the discovery of clinically relevant biomarker signatures. We designed a strategy for microarray analysis that is based on the identification of transcriptional modules formed by genes coordinately expressed in multiple disease data sets. Mapping changes in gene expression at the module level generated disease-specific transcriptional fingerprints that provide a stable framework for the visualization and functional interpretation of microarray data. These transcriptional modules were used as a basis for the selection of biomarkers and the development of a multivariate transcriptional indicator of disease progression in patients with systemic lupus erythematosus. Thus, this work describes the implementation and application of a methodology designed to support systems-scale analysis of the human immune system in translational research settings.
Figures
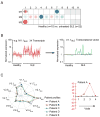
Figure 1. Analysis of patient blood leukocyte transcriptional profiles
A) Module-level analysis: Gene expression from patients with acute S. pneumoniae infection and respective healthy volunteer PBMCs were compared (p<0.05, Mann-Whitney U test) in modules M1.3, M1.5, M1.8 and M3.2. Pie charts indicate the proportion of genes significantly changed for each module. Graphs represent transcriptional profiles of genes that were significantly changed. Each line shows levels of expression (y-axis) of a single transcript across multiple conditions (samples, x-axis). Expression is normalized to the median expression value of the control group. Results obtained for the 28 PBMC transcriptional modules are displayed on a grid. Coordinates indicate module IDs (e.g. M2.8 is row M2, column 8). Spots indicate proportion of genes significantly changed for each module in patient with S. pneumoniae infection as compared to healthy controls. Red: overexpressed, Blue: underexpressed. B) Disease Fingerprints: Three additional datasets were similarly processed. Profiles were obtained from patients with Systemic Lupus Erythematosus, Liver transplant recipients under pharamacological immunosuppression infection and patients with metastatic melanoma. Functional interpretation is indicated on a grid by a color code. Detailed functional module descriptions are in Table 1.
Figure 2. Module-based biomarker selection strategy
Modules are used as a starting point for the generation of biomarker signatures and progressive reduction of the dimension of microarray data: A) Mapping global transcriptional changes using a modular framework identified 11 modules for which at least 15% of the transcripts are significantly changed between controls and SLE. B) Transcriptional vectors were generated by averaging the normalized expression values of differentially expressed transcripts for each one of the 11 modules selected. C) Composite expression values are plotted as vectors on a “spider graph”. Each line represents a patient profile. Multivariate scores can be generated to recapitulate the changes registered by several transcriptional vectors and monitor changes in an individual patient over time.
Figure 3. SLE transcriptional vectors
A) Composite transcriptional vectors identified from a pediatric SLE patient population sampled prior to the initiation of therapy. Each line on the radar plot represents a patient profile (logarithmic scale). Values are normalized per-gene using the median expression value of healthy. The thicker line represents the average normalized expression profile for this group of patients. Profiles generated for healthy volunteers B) and an independent cohort of pediatric SLE patients under treatment C). Averaged normalized expression profiles for treated (green) and untreated (orange) SLE patients cohorts D). Patient profiles were plotted on the same vectors on the basis of clinical activity (SLEDAI), regardless of treatment. E) Patients with low disease activity (SLEDAI from 0 to 6). F) Patients with high disease activity (SLEDAI from 14 to 28).
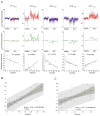
Figure 4. SLE transcriptional vectors correlate with disease activity
A) Expression profiles of genes forming vectors V1.7SLE, V2.2SLE, V2.4SLE, V2.8SLE and V3.1SLE correlating with a clinical SLE disease activity index (SLEDAI). Graphs represent expression level of individual transcripts forming each of the vectors in 12 healthy individuals and 21 untreated pediatric SLE patients. Average expression values across transcripts forming each vector are shown on the graph in yellow. Correlations between averaged vector expression values and SLEDAI are shown below (Spearman correlation). B) Multivariate scores were obtained for 22 untreated pediatric SLE patients by linear regression analysis of multivariate transcriptional U-scores (y axis) for vector V1.7SLE, V2.2SLE, V2.4SLE, V2.8SLE, V3.1 SLE, and SLEDAI (x axis). The light shaded area indicates the 95% limits confidence limits for individual predicted values. The dark shaded area indicates the 95% limits confidence limits for the slope and intercept. C) The same analysis applied to 31 pediatric SLE patients receiving different combinations of therapy.
Figure 5. Longitudinal disease monitoring with a multivariate disease activity score
SLEDAI index (blue, right y axis) and transcriptional U-scores (red, left y axis) of pediatric patients (identified by an SLE ID) over time (x axis). Time elapsed between sampling is indicated in months.
Figure 6. Cross-microarray platform comparison
PBMC samples from healthy donors and liver transplant recipient analyzed on two different microarray platforms: Affymetrix U133A&B GeneChips and Illumina Sentrix Human Ref8 BeadChips. The same source of total RNA was used to independently prepare biotin-labeled cRNA targets. Expression is normalized to the median of measurements obtained across all samples. Averaged expression values of the genes in each module are shown for both Affymetrix and Illumina platforms.
Comment in
- Bottom up: a modular view of immunology.
Wang E, Marincola FM. Wang E, et al. Immunity. 2008 Jul 18;29(1):9-11. doi: 10.1016/j.immuni.2008.07.002. Immunity. 2008. PMID: 18631452 Free PMC article.
Similar articles
- Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures.
Chiche L, Jourde-Chiche N, Whalen E, Presnell S, Gersuk V, Dang K, Anguiano E, Quinn C, Burtey S, Berland Y, Kaplanski G, Harle JR, Pascual V, Chaussabel D. Chiche L, et al. Arthritis Rheumatol. 2014 Jun;66(6):1583-95. doi: 10.1002/art.38628. Arthritis Rheumatol. 2014. PMID: 24644022 Free PMC article. - Key genes and functional coexpression modules involved in the pathogenesis of systemic lupus erythematosus.
Yan S, Wang W, Gao G, Cheng M, Wang X, Wang Z, Ma X, Chai C, Xu D. Yan S, et al. J Cell Physiol. 2018 Nov;233(11):8815-8825. doi: 10.1002/jcp.26795. Epub 2018 May 28. J Cell Physiol. 2018. PMID: 29806703 - Gene expression studies in systemic lupus erythematosus.
Mandel M, Achiron A. Mandel M, et al. Lupus. 2006;15(7):451-6. doi: 10.1191/0961203306lu2332oa. Lupus. 2006. PMID: 16898181 Review. - Genomic Investigation of Lupus in the Skin.
Sinha AA, Dey-Rao R. Sinha AA, et al. J Investig Dermatol Symp Proc. 2017 Oct;18(2):S75-S80. doi: 10.1016/j.jisp.2016.09.002. J Investig Dermatol Symp Proc. 2017. PMID: 28941499 Review.
Cited by
- GeVaDSs - decision support system for novel Genetic Vaccine development process.
Blazewicz J, Borowski M, Chaara W, Kedziora P, Klatzmann D, Lukasiak P, Six A, Wojciechowski P. Blazewicz J, et al. BMC Bioinformatics. 2012 May 10;13:91. doi: 10.1186/1471-2105-13-91. BMC Bioinformatics. 2012. PMID: 22574945 Free PMC article. - Applications of systems approaches in the study of rheumatic diseases.
Kim KJ, Lee S, Kim WU. Kim KJ, et al. Korean J Intern Med. 2015 Mar;30(2):148-60. doi: 10.3904/kjim.2015.30.2.148. Epub 2015 Feb 27. Korean J Intern Med. 2015. PMID: 25750554 Free PMC article. Review. - An ANN model for the differential diagnosis of tuberculosis and sarcoidosis.
Vijayaraj M, Abhinand PA, Venkatesan P, Ragunath PK. Vijayaraj M, et al. Bioinformation. 2020 Jul 31;16(7):539-546. doi: 10.6026/97320630016539. eCollection 2020. Bioinformation. 2020. PMID: 32994679 Free PMC article. - Gene signature-based mapping of immunological systems and diseases.
Liu H, Liu J, Toups M, Soos T, Arendt C. Liu H, et al. BMC Bioinformatics. 2016 Apr 18;17:171. doi: 10.1186/s12859-016-1012-y. BMC Bioinformatics. 2016. PMID: 27089880 Free PMC article. - Mapping systemic lupus erythematosus heterogeneity at the single-cell level.
Nehar-Belaid D, Hong S, Marches R, Chen G, Bolisetty M, Baisch J, Walters L, Punaro M, Rossi RJ, Chung CH, Huynh RP, Singh P, Flynn WF, Tabanor-Gayle JA, Kuchipudi N, Mejias A, Collet MA, Lucido AL, Palucka K, Robson P, Lakshminarayanan S, Ramilo O, Wright T, Pascual V, Banchereau JF. Nehar-Belaid D, et al. Nat Immunol. 2020 Sep;21(9):1094-1106. doi: 10.1038/s41590-020-0743-0. Epub 2020 Aug 3. Nat Immunol. 2020. PMID: 32747814 Free PMC article.
References
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. - PubMed
- Bae SC, Koh HK, Chang DK, Kim MH, Park JK, Kim SY. Reliability and validity of systemic lupus activity measure-revised (SLAM-R) for measuring clinical disease activity in systemic lupus erythematosus. Lupus. 2001;10:405–409. - PubMed
- Bammler T, Beyer RP, Bhattacharya S, Boorman GA, Boyles A, Bradford BU, Bumgarner RE, Bushel PR, Chaturvedi K, Choi D, et al. Standardizing global gene expression analysis between laboratories and across platforms. Nat Methods. 2005;2:351–356. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI068842/AI/NIAID NIH HHS/United States
- P50 AR055503/AR/NIAMS NIH HHS/United States
- P01 CA084512/CA/NCI NIH HHS/United States
- R01 AR050770/AR/NIAMS NIH HHS/United States
- U19 AI057234/AI/NIAID NIH HHS/United States
- R01 I068842/PHS HHS/United States
- P50 AR054083/AR/NIAMS NIH HHS/United States
- R01 CA078846/CA/NCI NIH HHS/United States
- U19 AIO57234-02/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases