The vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid improves the antitumor efficacy and shortens treatment time associated with Photochlor-sensitized photodynamic therapy in vivo - PubMed (original) (raw)
The vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid improves the antitumor efficacy and shortens treatment time associated with Photochlor-sensitized photodynamic therapy in vivo
Mukund Seshadri et al. Photochem Photobiol. 2009 Jan-Feb.
Abstract
In this report, we examined the antitumor activity of photodynamic therapy (PDT) in combination with 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a vascular disrupting agent currently undergoing clinical evaluation. BALB/c mice bearing subcutaneous CT-26 colon carcinomas were treated with PDT using the second-generation chlorin-based sensitizer, 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (Photochlor) with or without DMXAA. Long-term (60-days) treatment outcome, induction of tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6), vascular damage (microvessel density, MVD) were evaluated as endpoints. In addition, treatment selectivity was evaluated using magnetic resonance imaging (MRI) and the foot response assay. A highly synergistic interaction was observed with the combination of low-dose DMXAA and PDT (48 J cm(-2) at 112 mW cm(-2)) resulting in approximately 60% long-term cures. The duration of the PDT session for this combination therapy protocol was only 7 min, while the duration of a monotherapy PDT session, selected to yield the equivalent cure rate, was 152 min. MRI showed markedly less peritumoral edema after DMXAA + short-duration PDT compared with long-duration PDT monotherapy. Similarly, DMXAA + PDT caused significantly less phototoxicity to normal mouse foot tissue than PDT alone. Increased induction of cytokines TNF-alpha and IL-6 (P < 0.001) was observed at 4 h followed by extensive vascular damage, demonstrated by a significant reduction in MVD at 24 h after combination treatment. In conclusion, Photochlor-sensitized PDT in combination with DMXAA exhibits superior efficacy and improved selectivity with clinically feasible illumination schemes. Clinical evaluation of this novel combination strategy is currently being planned.
Figures
Figure 1
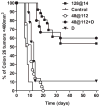
Long-term response of CT-26 murine colon carcinomas to low-dose DMXAA, HPPH-sensitized PDT and PDT–DMXAA combination therapy. Kaplan–Meier survival curves of BALB/c mice bearing subcutaneous CT-26 tumors treated with low-dose DMXAA (D; 25 mg kg−1, n = 9) alone, HPPH-PDT alone (48 at 112, n = 21 and 128 at 14, n = 21), HPPH-PDT in combination with DMXAA (48 at 112 + D, n = 15) or no treatment (control, n = 10). Log rank test P < 0.001 between 48 at 112 + D vs controls, D and 48 at 112.
Figure 2
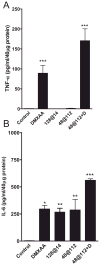
Induction of TNF-α and IL-6 in CT-26 tumors following HPPH-PDT, low-dose DMXAA and PDT–DMXAA combination therapy. TNF-α and IL-6 levels in tumors were determined 4 h after treatment using the ELISA. Data represent mean ± SE; n = 3–6 mice per group. *P < 0.05, **P < 0.01, P < 0.001 vs controls.
Figure 3
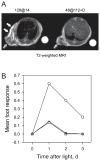
MRI and response of normal mouse foot tissue to PDT, DMXAA and combination treatment. (A) T2-weighted axial MR images acquired 4 h following treatment with PDT alone (128 at 14) and PDT + DMXAA (48 at 112 + D). Beveled arrows indicate hypointense regions within the tumor suggestive of treatment-induced vascular damage. Significant peritumoral tissue damage and edema demonstrated by hyperintense regions (white arrows) in the images was seen following treatment with PDT alone (using the low irradiance regimen) compared with PDT + DMXAA (using the high irradiance regimen) highlighting the selectivity of combination therapy. (B) Foot responses were graded according to the scale described in Materials and Methods using five mice/point following treatment with PDT using the high irradiance regimen, 48 at 112 (triangles), low irradiance regimen, 128 at 14 (circles) and combination of 48 at 112 and low-dose DMXAA (squares).
Similar articles
- Aminolevulinic acid-photodynamic therapy combined with topically applied vascular disrupting agent vadimezan leads to enhanced antitumor responses.
Marrero A, Becker T, Sunar U, Morgan J, Bellnier D. Marrero A, et al. Photochem Photobiol. 2011 Jul-Aug;87(4):910-9. doi: 10.1111/j.1751-1097.2011.00943.x. Epub 2011 Jun 13. Photochem Photobiol. 2011. PMID: 21575001 Free PMC article. - Tumor vascular response to photodynamic therapy and the antivascular agent 5,6-dimethylxanthenone-4-acetic acid: implications for combination therapy.
Seshadri M, Spernyak JA, Mazurchuk R, Camacho SH, Oseroff AR, Cheney RT, Bellnier DA. Seshadri M, et al. Clin Cancer Res. 2005 Jun 1;11(11):4241-50. doi: 10.1158/1078-0432.CCR-04-2703. Clin Cancer Res. 2005. PMID: 15930363 - 5,6-dimethylxanthenone-4-acetic acid (DMXAA): a new biological response modifier for cancer therapy.
Zhou S, Kestell P, Baguley BC, Paxton JW. Zhou S, et al. Invest New Drugs. 2002 Aug;20(3):281-95. doi: 10.1023/a:1016215015530. Invest New Drugs. 2002. PMID: 12201491 Review. - Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications.
Fan L, Jiang Z, Xiong Y, Xu Z, Yang X, Gu D, Ainiwaer M, Li L, Liu J, Chen F. Fan L, et al. Int J Mol Sci. 2023 Dec 12;24(24):17404. doi: 10.3390/ijms242417404. Int J Mol Sci. 2023. PMID: 38139233 Free PMC article. Review.
Cited by
- Chemophototherapy: An Emerging Treatment Option for Solid Tumors.
Luo D, Carter KA, Miranda D, Lovell JF. Luo D, et al. Adv Sci (Weinh). 2016 May 24;4(1):1600106. doi: 10.1002/advs.201600106. eCollection 2017 Jan. Adv Sci (Weinh). 2016. PMID: 28105389 Free PMC article. Review. - Enhancing photodynamyc therapy efficacy by combination therapy: dated, current and oncoming strategies.
Postiglione I, Chiaviello A, Palumbo G. Postiglione I, et al. Cancers (Basel). 2011 Jun 9;3(2):2597-629. doi: 10.3390/cancers3022597. Cancers (Basel). 2011. PMID: 24212824 Free PMC article. - Photodynamic therapy in gastroenterology.
Shishkova N, Kuznetsova O, Berezov T. Shishkova N, et al. J Gastrointest Cancer. 2013 Sep;44(3):251-9. doi: 10.1007/s12029-013-9496-4. J Gastrointest Cancer. 2013. PMID: 23645540 Review. - Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy.
Anand S, Wilson C, Hasan T, Maytin EV. Anand S, et al. Cancer Res. 2011 Sep 15;71(18):6040-50. doi: 10.1158/0008-5472.CAN-11-0805. Epub 2011 Aug 1. Cancer Res. 2011. PMID: 21807844 Free PMC article. - Aminolevulinic acid-photodynamic therapy combined with topically applied vascular disrupting agent vadimezan leads to enhanced antitumor responses.
Marrero A, Becker T, Sunar U, Morgan J, Bellnier D. Marrero A, et al. Photochem Photobiol. 2011 Jul-Aug;87(4):910-9. doi: 10.1111/j.1751-1097.2011.00943.x. Epub 2011 Jun 13. Photochem Photobiol. 2011. PMID: 21575001 Free PMC article.
References
- Dougherty TJ. A brief history of clinical photodynamic therapy at Roswell Park Cancer Institute. J Clin Laser Med Surg. 1996;14:219–221. - PubMed
- Hopper C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2001;1:212–219. - PubMed
- Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:147–157. - PubMed
- Foster TH, Hartley DF, Nichols MG, Hilf R. Fluence rate effects in photodynamic therapy of multicell tumor spheroids. Cancer Res. 1993;53:1249–1254. - PubMed
- Coutier S, Bezdetnaya LN, Foster TH, Parache RM, Guillemin F. Effect of irradiation fluence rate on the efficacy of photodynamic therapy and tumor oxygenation in metatetra-( hydroxyphenyl)chlorin (mTHPC)-sensitized HT29 xenografts in nude mice. Radiat Res. 2002;158:339–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016056/CA/NCI NIH HHS/United States
- CA16056/CA/NCI NIH HHS/United States
- R01CA89656/CA/NCI NIH HHS/United States
- R01 CA089656-09/CA/NCI NIH HHS/United States
- R01 CA089656/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources