Slug is a direct Notch target required for initiation of cardiac cushion cellularization - PubMed (original) (raw)
Slug is a direct Notch target required for initiation of cardiac cushion cellularization
Kyle Niessen et al. J Cell Biol. 2008.
Abstract
Snail family proteins are key regulators of epithelial-mesenchymal transition, but their role in endothelial-to-mesenchymal transition (EMT) is less well studied. We show that Slug, a Snail family member, is expressed by a subset of endothelial cells as well as mesenchymal cells of the atrioventricular canal and outflow tract during cardiac cushion morphogenesis. Slug deficiency results in impaired cellularization of the cardiac cushion at embryonic day (E)-9.5 but is compensated by increased Snail expression at E10.5, which restores cardiac cushion EMT. We further demonstrate that Slug, but not Snail, is directly up-regulated by Notch in endothelial cells and that Slug expression is required for Notch-mediated repression of the vascular endothelial cadherin promoter and for promoting migration of transformed endothelial cells. In contrast, transforming growth factor beta (TGF-beta) induces Snail but not Slug. Interestingly, activation of Notch in the context of TGF-beta stimulation results in synergistic up-regulation of Snail in endothelial cells. Collectively, our data suggest that combined expression of Slug and Snail is required for EMT in cardiac cushion morphogenesis.
Figures
Figure 1.
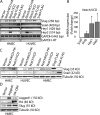
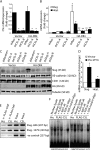
Expression of Slug, but not Snail, is induced by Notch activation. (A) Analysis of mRNA expression by semi–qRT-PCR in human mammary epithelial cells (HMEC) and human umbilical vein epithelial cells (HUVEC) expressing constitutively active Notch1 (Notch1ICD) or Notch4 (Notch4ICD). (B) Analysis of mRNA expression by qRT-PCR in HMEC expressing Notch1ICD. Results are normalized to the vector control (n = 3). *, P < 0.05. Error bars show SEM. (C) Immunoblots for Slug and Snail in HMEC, HUVEC, and human aortic endothelial cells (HAEC) transduced with Notch1ICD, Notch4ICD, or the empty vector. (D) HMEC expressing Jagged1 or Dll4 were cocultured with parental HMEC and immunoblotting for Slug was performed.
Figure 2.
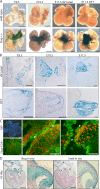
Slug expression during cardiac cushion development. (A) β-galactosidase staining (representing Slug expression) of whole-mounted hearts from Slug-lacZ+/− embryos from E9.5 to 11.5. Arrows point to the AV canal or OFT at E11.5. Bars, 250 μm. (B) Sections through the AV canal and OFT of β-galactosidase–stained Slug-lacZ+/− hearts from E9.5 to 11.5. Bars, 100 μm. (C) Immunofluorescence staining for Slug (red) and CD31 (green) in E11.5 embryonic mouse hearts. Arrowheads point to cells coexpressing Slug and CD31. Bars, 25 μm. (D) In situ hybridization for Snail and Slug in a 65-d human embryonic heart. Arrows point to the mitral and tricuspid valves, arrowheads indicate the interatrial septum, and the asterisk marks the AV septum. A higher magnification image of the heart valve is shown in the right panel of each analysis. Bars, 1 mm.
Figure 3.
_Slug_−/− embryos display defects in AV canal EMT at E9.5. (A) Phase contrast (left) and DAPI (right) images of AV canal explants from wild-type and _Slug_−/− embryos. Bars, 250 μm. (B) Quantitative analysis of EMT in AV canal explants from E9.5 wild-type (wt), Slug+/−, and _Slug_−/− embryos after 48 h in culture. Results represent the distance of a positive pixel (DAPI-stained nucleus) to the closest point of the AV canal normalized to the area of the AV canal tissue. *, P < 0.05. (C) Slug expression in the AV canal explant assay as visualized by β-galactosidase staining of wild-type and Slug-lacZ+/− AV explants. The rounded morphology of most of the LacZ+ cells is shown on the right. The black square indicates the region of higher magnification shown to the right. Bars, 50 μm. (D) Representative sections of wild-type and _Slug_−/− hearts counterstained with Nuclear Fast Red used for analysis in E. Dotted blue lines highlight the superior and inferior AV cushions. Bars, 50 μm. (E) Quantitation of the cellularity of the superior and inferior cushions in E9.5 wild-type (wt; n = 3) and Slug−/− (n = 3) embryos. Error bars show SEM. (F) BrdU analysis on the percentage of proliferating cells in wild-type (n = 4) and Slug−/− (n = 6) AV canal cardiac cushions (total), the AV canal endocardium (Endo), and AV canal mesenchymal cells (Mesen; 10–15 sections per embryo). Error bars show SEM. (G) Vector- or Slug-transduced HMEC were subjected to an endothelial wounding assay. Bars represent the distance migrated after 24 h (n = 4). *, P < 0.05. Error bars show SD. (H) Vector- or Slug-transduced HMEC were evaluated in a modified Boyden chamber assay with 20 ng/ml PDGF-BB present in the lower chamber. Bars represent the total number of cells migrated after 4 h (n = 6). *, P < 0.05. Error bars show SD.
Figure 4.
Slug represses the endothelial phenotype and directly regulates the VE-cadherin promoter. (A) Analysis of endothelial marker expression by qRT-PCR in Slug-transduced HMEC (n = 3). *, P < 0.05. (B) Immunoblots for endothelial and mesenchymal markers in empty vector–, NotchICD-, and Slug-expressing HMEC. (C) EMSA using in vitro–translated luciferase (Luc) or Slug-FLAG protein and 32P-labeled double-stranded oligonucleotides for an E-box cis element (−97) or two putative Slug E2-box motifs (−306 and −379) in the human VE-cadherin promoter. Supershift assays with anti–FLAG-M2 or IgG control antibodies and competition assays with 50× wild-type (wt) or mutant probes are also shown. (D) Promoter activity in endothelial cells cotransfected with vector or Slug plasmids and wild-type, E2-box mutant, or E-box mutant mouse VE-cadherin promoter-luciferase constructs (n = 4; each experiment performed in triplicate). *, P < 0.05. Error bars show SEM.
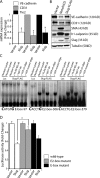
Figure 5.
Notch signaling regulates Slug expression through a CSL-dependent pathway. (A) qRT-PCR analysis demonstrating efficient knockdown of CSL in HMEC with two different shRNAs targeting CSL (shCSL) compared with a random control sequence (shRan). (B) qRT-PCR of Slug and HeyL in vector- or Dll4-activated HMEC transduced with shCSL constructs (n = 3). *, P < 0.05 vector shRandom versus HA-D114 shRandom; **, P < 0.05 HA-D114 shRandom versus HA-D114 shCSL-A or shCSL-B. (C) Immunoblotting for Slug, VE-cadherin, and CD31 in vector- or Dll4-activated HMEC transduced with shCSL or shSlug constructs. (D) qRT-PCR of vector- or CSL-VP16–expressing HMEC for Slug and HeyL (n = 3). *, P < 0.05. (E) PCR after ChIP with anti–FLAG-M2 antibody on HMEC-expressing vector (vec) or FLAG-CSL (CSL) to demonstrate CSL binding to the human Slug promoter. The negative (-ve) control represents PCR of the ZNF3 promoter after ChIP using FLAG-M2. (F) EMSA using nuclear lysates collected from vector- or FLAG-CSL–expressing HMEC and 32P-labeled double-stranded oligonucleotides spanning each of the two CSL binding sites in the human Slug promoter. Supershift assays with anti–FLAG-M2 or IgG control antibodies, and competition assays with 50× wild-type (wt) or mutant probes are also shown. Error bars show SEM.
Figure 6.
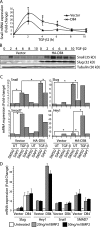
Induction of Snail by TGF-β2 is synergistically enhanced in Dll4-activated endothelial cells. (A) qRT-PCR for Snail mRNA in vector- or Dll4-activated HMEC treated with 2.5 ng/ml TGF-β2 for the indicated times (n = 3). *, P < 0.05. (B) Immunoblots for Snail and Slug in vector- or Dll4-activated HMEC treated with 2.5 ng/ml TGF-β2. (C) qRT-PCR for Snail, Slug, Hey1, and Smad7 mRNA in vector- or Dll4-activated HMEC treated with 10 μM DMSO or DAPT for 16 h followed by treatment with 2.5 ng/ml TGF-β2 for 3 h (n = 3). *, P < 0.05. (D) qRT-PCR for Snail, Slug, Hey1, and Smad7 mRNA in vector- or Dll4-activated HMEC treated with 20 or 50 ng/ml BMP2 (n = 3). Error bars show SEM.
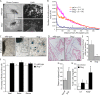
Figure 7.
Increased Snail expression compensates for Slug deficiency. (A) qRT-PCR analysis for Snail and Slug of whole hearts isolated from E9.5, 10.5, and 11.5 wild-type (wt) and _Slug_−/−embryos (n = 3). *, P < 0.05. Error bars show SEM. (B) In situ hybridization for Snail in E10.5 and 11.5 wild-type (wt) and _Slug_−/− hearts (n = 3; five embryos in each replicate). Bars, 250 μm. (C) Quantitation of EMT in AV canal explants from E9.5 wild-type (wt) or Slug+/− and _Slug_−/− embryos treated with 5 ng/ml TGF-β2 or vehicle (UT). (D) Quantitation of EMT in AV canal explants from E10.5 wild-type (wt) or Slug+/− and _Slug_−/− embryos transduced with shRandom or shSnail constructs.
Similar articles
- Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion.
Zhang H, von Gise A, Liu Q, Hu T, Tian X, He L, Pu W, Huang X, He L, Cai CL, Camargo FD, Pu WT, Zhou B. Zhang H, et al. J Biol Chem. 2014 Jul 4;289(27):18681-92. doi: 10.1074/jbc.M114.554584. Epub 2014 May 15. J Biol Chem. 2014. PMID: 24831012 Free PMC article. - Endocardial cushion morphogenesis and coronary vessel development require chicken ovalbumin upstream promoter-transcription factor II.
Lin FJ, You LR, Yu CT, Hsu WH, Tsai MJ, Tsai SY. Lin FJ, et al. Arterioscler Thromb Vasc Biol. 2012 Nov;32(11):e135-46. doi: 10.1161/ATVBAHA.112.300255. Epub 2012 Sep 6. Arterioscler Thromb Vasc Biol. 2012. PMID: 22962329 Free PMC article. - Mmp15 is a direct target of Snai1 during endothelial to mesenchymal transformation and endocardial cushion development.
Tao G, Levay AK, Gridley T, Lincoln J. Tao G, et al. Dev Biol. 2011 Nov 15;359(2):209-21. doi: 10.1016/j.ydbio.2011.08.022. Epub 2011 Sep 5. Dev Biol. 2011. PMID: 21920357 Free PMC article. - Nfatc1 directs the endocardial progenitor cells to make heart valve primordium.
Wu B, Baldwin HS, Zhou B. Wu B, et al. Trends Cardiovasc Med. 2013 Nov;23(8):294-300. doi: 10.1016/j.tcm.2013.04.003. Epub 2013 May 10. Trends Cardiovasc Med. 2013. PMID: 23669445 Free PMC article. Review. - Snail: More than EMT.
Wu Y, Zhou BP. Wu Y, et al. Cell Adh Migr. 2010 Apr-Jun;4(2):199-203. doi: 10.4161/cam.4.2.10943. Epub 2010 Apr 14. Cell Adh Migr. 2010. PMID: 20168078 Free PMC article. Review.
Cited by
- EMT reversal in human cancer cells after IR knockdown in hyperinsulinemic mice.
Zelenko Z, Gallagher EJ, Antoniou IM, Sachdev D, Nayak A, Yee D, LeRoith D. Zelenko Z, et al. Endocr Relat Cancer. 2016 Sep;23(9):747-58. doi: 10.1530/ERC-16-0142. Epub 2016 Jul 19. Endocr Relat Cancer. 2016. PMID: 27435064 Free PMC article. - Roles of transducin-like enhancer of split (TLE) family proteins in tumorigenesis and immune regulation.
Yu G, Chen Y, Hu Y, Zhou Y, Ding X, Zhou X. Yu G, et al. Front Cell Dev Biol. 2022 Nov 11;10:1010639. doi: 10.3389/fcell.2022.1010639. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36438567 Free PMC article. Review. - The Notch signaling pathway as a mediator of tumor survival.
Capaccione KM, Pine SR. Capaccione KM, et al. Carcinogenesis. 2013 Jul;34(7):1420-30. doi: 10.1093/carcin/bgt127. Epub 2013 Apr 12. Carcinogenesis. 2013. PMID: 23585460 Free PMC article. Review. - GRWD1 Over-Expression Promotes Gastric Cancer Progression by Activating Notch Signaling Pathway via Up-Regulation of ADAM17.
Ding H, Feng Z, Hu K. Ding H, et al. Dig Dis Sci. 2024 Mar;69(3):821-834. doi: 10.1007/s10620-023-08208-5. Epub 2024 Jan 3. Dig Dis Sci. 2024. PMID: 38172445 - SOX9 reprograms endothelial cells by altering the chromatin landscape.
Fuglerud BM, Drissler S, Lotto J, Stephan TL, Thakur A, Cullum R, Hoodless PA. Fuglerud BM, et al. Nucleic Acids Res. 2022 Aug 26;50(15):8547-8565. doi: 10.1093/nar/gkac652. Nucleic Acids Res. 2022. PMID: 35904801 Free PMC article.
References
- Bartram, U., D.G. Molin, L.J. Wisse, A. Mohamad, L.P. Sanford, T. Doetschman, C.P. Speer, R.E. Poelmann, and A.C. Gittenberger-de Groot. 2001. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 103:2745–2752. - PubMed
- Brown, C.B., A.S. Boyer, R.B. Runyan, and J.V. Barnett. 1999. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 283:2080–2082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials