The Galphai and Galphaq proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation - PubMed (original) (raw)
The Galphai and Galphaq proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation
Ling Lai et al. J Pineal Res. 2008 Nov.
Abstract
Melatonin, via its MT1 receptor, but not the MT2 receptor, can modulate the transcriptional activity of various nuclear receptors - estrogen receptor alpha (ERalpha) and retinoic acid receptor alpha (RARalpha), but not ERbeta- in MCF-7, T47D, and ZR-75-1 human breast cancer cell lines. The anti-proliferative and nuclear receptor modulatory actions of melatonin are mediated via the MT1 G protein-coupled receptor expressed in human breast cancer cells. However, the specific G proteins and associated pathways involved in the nuclear receptor transcriptional regulation by melatonin are not yet clear. Upon activation, the MT1 receptor specifically couples to the G(alphai2), G(alphai3), G(alphaq), and G(alphall) proteins, and via activation of G(alphai2) proteins, melatonin suppresses forskolin-induced 3',5'-cyclic adenosine monophosphate production, while melatonin activation of G(alphaq), is able to inhibit phospholipid hydrolysis and ATP's induction of inositol triphosphate production in MCF-7 breast cancer cells. Employing dominant-negative and dominant-positive) forms of these G proteins, we demonstrate that G(alphai2) proteins mediate the suppression of estrogen-induced ERalpha transcriptional activity by melatonin, while the G(q) protein mediates the enhancement of retinoid-induced RARalpha transcriptional activity by melatonin. However, the growth-inhibitory actions of melatonin are mediated via both G(alphai2) and G(alphaq) proteins.
Figures
Fig. 1
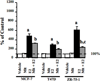
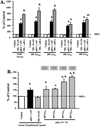
Effects of melatonin on ERα transcriptional activity in MCF-7, T47D and ZR-75-1 human breast cancer cells. MCF-7, T47D and ZR-75-1 breast cancer cells grown in phenol red-free medium supplemented with 5% CS-FBS and were used to examine ERα transcriptional activity using an ERE-luciferase reporter construct as described in Materials and Methods with vehicle (0.001% ethanol), 10 nM melatonin, 1 nM E2, or pretreated with melatonin for 30 min followed by E2. Luciferase activity was recorded as mean relative light units (RLUs). For comparison purposes between tumor cell lines diluent treated values were set at 100% and activity in response to other treatments was recorded as percent of control activity. n=3 independent experiments; a, _P < 0.05 vs. control; b, P < 0.0_5 vs. E2 alone.
Fig. 2
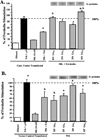
Effects of melatonin via MT1 and MT2 receptors on ERα and ERβ transcriptional activity in HEK293 embryonic kidney cells. HEK293 embryonic kidney cells were grown in phenol red-free medium supplemented with 5% CS-FBS. After 3 days in media supplemented with 5% CS-FBS cells were transiently transfected with ERα or ERβ, MT1 or MT2 cDNA expression vectors, an ERE-luciferase reporter construct and treated for 18 h with vehicle (0.001% ethanol), 10 nM melatonin, 1 nM E2, or pretreated with melatonin for 30 min followed by E2 as described in Materials and Methods. Luciferase activity was recorded as mean relative light units (RLUs). n=3 independent experiments; a, _P < 0.05 vs. control; b, P < 0.0_5 vs. E2 alone.
Fig. 3
Modulation of ERα phosphorylation in MCF-7 breast cancer cells by E2, melatonin and a DP-Gαi2 protein. Immunoblot analysis of phosphorylated ERα in response to E2 (1 nM), melatonin (10 nM) and expression of a DP-Gαi2 (50 mg/well) cDNA. Phosphorylated ERα is on the top band and total ERα is the bottom band.
Fig. 4
Expression of G proteins and MT1 receptor in MCF-7 breast cancer cells. (a) G-proteins expressed in MCF-7 cells. Seventy micrograms of total cellular protein from MCF-7 cells were loaded onto each lane. Specific G-proteins were detected using anti-G protein polyclonal rabbit antibodies (Gαi2, Gαi3, Gαq, Gα11, Gαo, Gαz, and Gα12), anti-Gα16 goat polyclonal antibodies, or anti-Gαi1 mouse monoclonal antibody at a 1:1000 dilution. (b) MT1-MCF-7 cells were transiently transfected with wild-type G-protein (Gαi2, Gαi3, Gαq, Gα11, Gαo, Gαz, Gα12, Gα16 and Gαi1) expression vectors for 24 h, followed by treatment with 10 nM melatonin. Lysates were immunoprecipitated with the anti-MT1 536 and protein A-agarose beads. (c) The extracts from the MT1-MCF-7 cells treated with either control vehicle (C) [0.001% alcohol] or 1 nM melatonin (Mlt) for 30 min. were incubated with anti-MT1 536 antibody for immunoprecipition. G-proteins coupled to the MT1 receptor were detected by immunoblot blot analysis, using specific anti-G-protein antibodies as described in Materials and Methods.
Fig. 5
Modulation of forskolin-stimulated cAMP accumulation by melatonin in MCF-7 cells with transient expression of G-protein. (a) Modulation of forskolin-stimulated cAMP accumulation by melatonin in the cells expressing DN-G-proteins. Cells were transfected transiently with DN-G-protein expression constructs then treated as described in Materials and Methods. Cell extracts (1:1000 dilution) were analysized for cAMP levels using the cAMP-[125I] assay system as described in Materials and Methods. (b) Modulation of forskolin-stimulated cAMP accumulation in the cells expressing DP-G-proteins. Cell extracts (1:1000 dilution) were analyzed for cAMP levels as described above. Expression of DN-G-proteins and DP-G-proteins following transfection was evaluated by immunoblot analysis of duplicate cell lysates and expression is shown above the bar graph. Results are expressed as a percentage of Fsk stimulation. (n =3 experiments in triplicate for each group). a, P < 0.05 vs. control, b, P < 0.05 vs. Fsk).
Fig. 6
Modulation of ATP-stimulated IP3 levels by melatonin in MCF-7 cells. MCF-7 cells were transfected with DN-G proteins (a) or DP-G proteins [Gαi2, Gαi3, Gαq and Gll] (b) and treated with ATP. Following the plating, the cells were incubated for 20 min in 10 mM LiCl, and then treated as described in Materials and Methods. Cell lysates were analyzed using the Inositol-1,4,5-triphosphate (IP3) [3H] assay system as described in Materials and Methods. Expression of DP-G-proteins following transfection was evaluated by immunoblot analysis of duplicate cell lysates and expression is shown above the appropriate bar graphs. The data is presented as the mean IP3 (pmol/tube) × 10−2 ± S.E.M. n=3 independent experiments; a, _P < 0.05 vs. control; b, P < 0.0_5 vs. ATP.
Fig. 7
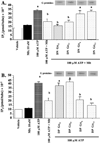
Effects of G-proteins on ERα transcriptional activity in MCF-7 cells. MCF-7 cells were transiently transfected with an ERE-luciferase reporter construct and DN-G-protein plasmids. (a) Cells were treated with vehicle (0.001% ethanol), 10 nM melatonin, 1 nM E2, or pretreated with melatonin for 30 min followed by E2 and harvested for luciferase assay. (b) Effects of on melatonin-mediated inhibition of ERα transcriptional activity in MCF-7 cells. Cells were treated as described above, but transfected with DP-G-protein plasmids. Expression of DP-G-proteins following transfection was evaluated by immunoblot analysis of duplicate cell lysates and expression is shown above the bar graphs. For comparison purposes vector control diluent treated values were set at 100% and activity in response to other treatments was recorded as percent of control activity. n=3 independent experiments; a, _P < 0.05 vs. control; b, P < 0.0_5 vs. E2 alone.
Fig. 8
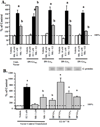
Effects of melatonin and DN/DP-G-proteins on RARα transcriptional activity in MCF-7 cells. (a) Effects of DN-G-proteins on melatonin-mediated enhancement of _at_RA-induced RARα transcriptional activity in MCF-7 cells. Cells were transiently transfected with RARE-luciferase reporter construct and DN-G-protein plasmids. Cells in medium supplemented with 5% CS-FBS were treated for with vehicle (0.001% ethanol), 10 nM melatonin, 1 nM _at_RA, or melatonin and _at_RA simultaneously. (b) Effects of DP-G-proteins on melatonin-mediated enhancement of RARα transcriptional activity in MCF-7 cells. Cells were transfected with an RARE as described above and DP-G-protein plasmids and treated with either vehicle (0.001% ethanol) 10 nM melatonin or 1 nM _at_RA. Expression of DP-G-proteins was evaluated by immunoblot analysis is shown above the bar graphs. For comparison purposes vector control diluent treated values were set at 100% and activity in response to other treatments was recorded as percent of control activity. n=3 independent experiments; a, P < 0.05 vs. control; b, P < 0.05 vs. _at_RA-stimulated vector controls.
Fig. 9
Growth-inhibitory effect of melatonin regulated by Gai2 and Gaq proteins in MCF-7 cells. The MCF-7 cells were transiently transfected with control vector or (a) DN-G-protein plasmids for 8 h and then treated with 0.001% ethanol or 10 nM melatonin for 7 days; (b) MCF-7 cells were transfected with DP-G-protein plasmids for and then treated with 0.001% ethanol for 7 days. Control cells include diluent treated controls (0.001% ethanol) and melatonin (10 nM) treated controls. Expression of DP-G-proteins was evaluated by immunoblot analysis is shown above the bar graphs. Viable cells as measured by trypan blue exclusion were counted using a hemocytometer. The results represent the mean cell number × 104/well ± S.E.M. of data from at least three independent experiments each performed in triplicate; a, _P < 0.05 vs. control; b, P < 0.0_5 vs. melatonin treated.
Similar articles
- Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night.
Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, Mao L, Dauchy E, Sauer LA. Blask DE, et al. J Pineal Res. 2011 Oct;51(3):259-69. doi: 10.1111/j.1600-079X.2011.00888.x. Epub 2011 May 24. J Pineal Res. 2011. PMID: 21605163 Free PMC article. Review. - Molecular mechanisms of melatonin anticancer effects.
Hill SM, Frasch T, Xiang S, Yuan L, Duplessis T, Mao L. Hill SM, et al. Integr Cancer Ther. 2009 Dec;8(4):337-46. doi: 10.1177/1534735409353332. Integr Cancer Ther. 2009. PMID: 20050373 - Age-related decline in melatonin and its MT1 receptor are associated with decreased sensitivity to melatonin and enhanced mammary tumor growth.
Hill SM, Cheng C, Yuan L, Mao L, Jockers R, Dauchy B, Blask DE. Hill SM, et al. Curr Aging Sci. 2013 Feb;6(1):125-33. doi: 10.2174/1874609811306010016. Curr Aging Sci. 2013. PMID: 23895529 Review.
Cited by
- Novel Melatonin, Estrogen, and Progesterone Hormone Therapy Demonstrates Anti-Cancer Actions in MCF-7 and MDA-MB-231 Breast Cancer Cells.
Hasan M, Browne E, Guarinoni L, Darveau T, Hilton K, Witt-Enderby PA. Hasan M, et al. Breast Cancer (Auckl). 2020 Jun 24;14:1178223420924634. doi: 10.1177/1178223420924634. eCollection 2020. Breast Cancer (Auckl). 2020. PMID: 32636633 Free PMC article. - Melatonin and Hippo Pathway: Is There Existing Cross-Talk?
Lo Sardo F, Muti P, Blandino G, Strano S. Lo Sardo F, et al. Int J Mol Sci. 2017 Sep 6;18(9):1913. doi: 10.3390/ijms18091913. Int J Mol Sci. 2017. PMID: 28878191 Free PMC article. - Molecular mechanisms of melatonin's inhibitory actions on breast cancers.
Proietti S, Cucina A, Reiter RJ, Bizzarri M. Proietti S, et al. Cell Mol Life Sci. 2013 Jun;70(12):2139-57. doi: 10.1007/s00018-012-1161-8. Epub 2012 Sep 25. Cell Mol Life Sci. 2013. PMID: 23007844 Free PMC article. Review. - Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night.
Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, Mao L, Dauchy E, Sauer LA. Blask DE, et al. J Pineal Res. 2011 Oct;51(3):259-69. doi: 10.1111/j.1600-079X.2011.00888.x. Epub 2011 May 24. J Pineal Res. 2011. PMID: 21605163 Free PMC article. Review. - Molecular deficiency (ies) in MT₁ melatonin signaling pathway underlies the melatonin-unresponsive phenotype in MDA-MB-231 human breast cancer cells.
Mao L, Yuan L, Xiang S, Zeringue SB, Dauchy RT, Blask DE, Hauch A, Hill SM. Mao L, et al. J Pineal Res. 2014 Apr;56(3):246-53. doi: 10.1111/jpi.12117. Epub 2014 Jan 18. J Pineal Res. 2014. PMID: 24372669 Free PMC article.
References
- Hill SM, Blask DE. Effects of the pineal hormone on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1998;48:6121–6126. - PubMed
- Yuan L, Collins AR, Dai J, Dubocovich ML, Hill SM. MT1 melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol Cell Endocrinol. 2002;192:147–156. - PubMed
- Bourne HR, Landis CA, Masters SB. Hydrolysis of GTP by the alpha chain of GS and other GTP binding proteins. Proteins. 1989;6:222–230. - PubMed
- Neer EJ, Clapham DE. Roles of G protein subunits in transmembrane signaling. Nature. 1988;333:129–134. - PubMed
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimmers as well as alpha subunits. Cell. 1992;71:1069–1072. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical