The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae - PubMed (original) (raw)
The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae
Camilla A Hansson Petersen et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The amyloid beta-peptide (Abeta) has been suggested to exert its toxicity intracellularly. Mitochondrial functions can be negatively affected by Abeta and accumulation of Abeta has been detected in mitochondria. Because Abeta is not likely to be produced locally in mitochondria, we decided to investigate the mechanisms for mitochondrial Abeta uptake. Our results from rat mitochondria show that Abeta is transported into mitochondria via the translocase of the outer membrane (TOM) machinery. The import was insensitive to valinomycin, indicating that it is independent of the mitochondrial membrane potential. Subfractionation studies following the import experiments revealed Abeta association with the inner membrane fraction, and immunoelectron microscopy after import showed localization of Abeta to mitochondrial cristae. A similar distribution pattern of Abeta in mitochondria was shown by immunoelectron microscopy in human cortical brain biopsies obtained from living subjects with normal pressure hydrocephalus. Thus, we present a unique import mechanism for Abeta in mitochondria and demonstrate both in vitro and in vivo that Abeta is located to the mitochondrial cristae. Importantly, we also show that extracellulary applied Abeta can be internalized by human neuroblastoma cells and can colocalize with mitochondrial markers. Together, these results provide further insight into the mitochondrial uptake of Abeta, a peptide considered to be of major significance in Alzheimer's disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
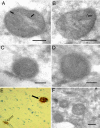
Immunoelectron microscopy of Aβ1-42 localization to mitochondria in a human brain biopsy from patient #1 with amyloidosis using JNAβ1-42 antibody (A and B). Arrows indicate ImmunoGold labeling. Bars: 0.2 μm. Preabsorption of antibody with Aβ1-42 peptide abolished labeling of mitochondria: control (C), preabsorption (D). Bars: 0.2 μm. Protein aggregates in the same frontal cortex biopsy (patient #1) as in A to D visualized by immonohistochemistry applying antibody directed to β-amyloid (clone 6F/3D). Magnification 200×. Both cerebral amyloid angiopathy (open arrow) and a dense aggregate (black arrow) are seen (E). Immunoelectron microscopy of Aβ1-42 localization to mitochondria in a human brain biopsy from patient #3 with tauopathy, using JNAβ1-42 antibody (F). Bar: 0.2 μm.
Fig. 2.
Confocal immunofluorescence microscopy analysis of human neuroblastoma SH-SY5Y cells treated with 1 μM Aβ1-40-HiLyte Fluor (Alexa Fluor 488) for 18 h. DAPI stains the nuclei and MitoTracker stains mitochondria. The yellow color in the merged image indicates overlap between green (Aβ1-40-HiLyte Fluor) and red (MitoTracker Orange) fluorescence.
Fig. 3.
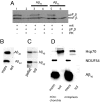
In vitro import of Aβ1-40 (A), Aβ1-42 (B), and pF1β (C) detected by immunoblot analysis. Aβ peptides and F1β (pF1β = precursor F1β; mF1β = mature F1β) were imported into isolated mitochondria from rat liver followed by treatment with PK. Valinomycin (Val), CyclosporinA (CsA), antibodies raised toward Tom70, Tom20, Tom40, and VDAC were used as described in Materials and Methods to investigate the import mechanism of Aβ1-40 and Aβ1-42. In some experiments, mitochondria were pretreated with Proteinase K (PKpretreatment) before import. The degradation of mitochondrial receptors was analyzed using antibodies toward Tom20 and Tim23 (D).
Fig. 4.
Analysis of Aβ localization after in vitro import. pF1β import analysis by phosphoimaging following Aβ1-40 and Aβ1-42 import (A). Mitochondria were fractionated after Aβ import and Proteinase K treatment into a soluble (sol) and a membrane (mem) fraction followed by immunoblot analysis (B). The membrane fraction in (B) was further treated with 0.1-mM Na2CO3 followed by immunoblot analysis (C). Mitochondria and mitoplasts were fractionated into a soluble (sol) and a membrane (mem) fraction after Aβ1-40 import. Grp75 is a mitochondrial matrix marker and Ndufs is an mitochondrial inner membrane marker (D).
Fig. 5.
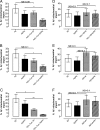
Isolated mitochondria were treated in the presence or absence of proteinase K (PK). MitoFluor Red positive mitochondria were selected and gated for by flow cytometry. A significant inhibition of Aβ/α-Tom20/PK, Aβ/α-Tom40/PK, and Aβ/α-Tom70/PK (*, P < 0.05, *, P < 0.05, **, P < 0.01) as compared to Aβ/PK treated mitochondria is shown (A–C). Pretreatment of mitochondria with VDAC antibody, valinomycin (Val) or cyclosporine A (CsA) had no statistically significant effects on Aβ1-40 import (D–F).
Fig. 6.
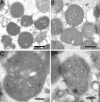
Immunoelectron microscopy of in vitro imported Aβ1-42 using JNAβ1-42 antibody. Mitochondria without Aβ1-42 in the import assay (A). Mitochondria after Aβ1-42 import (B–D). Bars: 2 μm (A and B); 0.2 μm (C and D).
Similar articles
- Chronic exposure to sub-lethal beta-amyloid (Abeta) inhibits the import of nuclear-encoded proteins to mitochondria in differentiated PC12 cells.
Sirk D, Zhu Z, Wadia JS, Shulyakova N, Phan N, Fong J, Mills LR. Sirk D, et al. J Neurochem. 2007 Dec;103(5):1989-2003. doi: 10.1111/j.1471-4159.2007.04907.x. Epub 2007 Sep 13. J Neurochem. 2007. PMID: 17868329 - Mitochondrial import and degradation of amyloid-β peptide.
Pinho CM, Teixeira PF, Glaser E. Pinho CM, et al. Biochim Biophys Acta. 2014 Jul;1837(7):1069-74. doi: 10.1016/j.bbabio.2014.02.007. Epub 2014 Feb 18. Biochim Biophys Acta. 2014. PMID: 24561226 Review. - Mitochondrial accumulation of amyloid β (Aβ) peptides requires TOMM22 as a main Aβ receptor in yeast.
Hu W, Wang Z, Zheng H. Hu W, et al. J Biol Chem. 2018 Aug 17;293(33):12681-12689. doi: 10.1074/jbc.RA118.002713. Epub 2018 Jun 20. J Biol Chem. 2018. PMID: 29925587 Free PMC article. - Amyloid β-peptides interfere with mitochondrial preprotein import competence by a coaggregation process.
Cenini G, Rüb C, Bruderek M, Voos W. Cenini G, et al. Mol Biol Cell. 2016 Nov 1;27(21):3257-3272. doi: 10.1091/mbc.E16-05-0313. Epub 2016 Sep 14. Mol Biol Cell. 2016. PMID: 27630262 Free PMC article. - Mechanisms and pathways of mitochondrial outer membrane protein biogenesis.
Gupta A, Becker T. Gupta A, et al. Biochim Biophys Acta Bioenerg. 2021 Jan 1;1862(1):148323. doi: 10.1016/j.bbabio.2020.148323. Epub 2020 Oct 7. Biochim Biophys Acta Bioenerg. 2021. PMID: 33035511 Review.
Cited by
- Amylovis-201 is a new dual-target ligand, acting as an anti-amyloidogenic compound and a potent agonist of the σ 1 chaperone protein.
García-Pupo L, Crouzier L, Bencomo-Martínez A, Meunier J, Morilleau A, Delprat B, Carrazana MS, Menéndez Soto Del Valle R, Maurice T, Rodríguez-Tanty C. García-Pupo L, et al. Acta Pharm Sin B. 2024 Oct;14(10):4345-4359. doi: 10.1016/j.apsb.2024.06.013. Epub 2024 Jun 20. Acta Pharm Sin B. 2024. PMID: 39525570 Free PMC article. - The potential role of gut microbiota-derived metabolites as regulators of metabolic syndrome-associated mitochondrial and endolysosomal dysfunction in Alzheimer's disease.
Jung YH, Chae CW, Han HJ. Jung YH, et al. Exp Mol Med. 2024 Aug;56(8):1691-1702. doi: 10.1038/s12276-024-01282-3. Epub 2024 Aug 1. Exp Mol Med. 2024. PMID: 39085351 Free PMC article. Review. - Astrocytes in Amyloid Generation and Alcohol Metabolism: Implications of Alcohol Use in Neurological Disorder(s).
Kumar M, Swanson N, Ray S, Buch S, Saraswathi V, Sil S. Kumar M, et al. Cells. 2024 Jul 10;13(14):1173. doi: 10.3390/cells13141173. Cells. 2024. PMID: 39056755 Free PMC article. - Metabolic regulation of misfolded protein import into mitochondria.
Wang Y, Ruan L, Zhu J, Zhang X, Chang AC, Tomaszewski A, Li R. Wang Y, et al. Elife. 2024 Jun 20;12:RP87518. doi: 10.7554/eLife.87518. Elife. 2024. PMID: 38900507 Free PMC article.
References
- Vassar R, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. - PubMed
- Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. - PubMed
- Soderberg L, et al. Analysis of single Alzheimer solid plaque cores by laser capture microscopy and nanoelectrospray/tandem mass spectrometry. Biochemistry. 2006;45:9849–9856. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources