Depletion of the ATPase NSF from Golgi membranes with hypo-S-nitrosylation of vasorelevant proteins in endothelial cells exposed to monocrotaline pyrrole - PubMed (original) (raw)
Depletion of the ATPase NSF from Golgi membranes with hypo-S-nitrosylation of vasorelevant proteins in endothelial cells exposed to monocrotaline pyrrole
Somshuvra Mukhopadhyay et al. Am J Physiol Heart Circ Physiol. 2008 Nov.
Abstract
Investigations of regulated S-nitrosylation and denitrosylation of vasorelevant proteins are a newly emergent area in vascular biology. We previously showed that monocrotaline pyrrole (MCTP)-induced megalocytosis of pulmonary arterial endothelial cells (PAECs), which underlies the development of pulmonary arterial hypertension, was associated with a Golgi blockade characterized by the trapping of diverse vesicle tethers, soluble N-ethylmaleimide-sensitive factor (NSF)-attachment protein receptors (SNAREs), and soluble NSF-attachment proteins (SNAPs) in the Golgi; reduced trafficking of caveolin-1 (cav-1) and endotheial nitric oxide (NO) synthase (eNOS) from the Golgi to the plasma membrane; and decreased caveolar NO. We have investigated whether NSF, the ATPase involved in all SNARE disassembly, might be the upstream target of MCTP and whether MCTP might regulate NSF by S-nitrosylation. Immunofluorescence microscopy and Golgi purification techniques revealed the discordant decrease of NSF by approximately 50% in Golgi membranes after MCTP despite increases in alpha-SNAP, cav-1, eNOS, and syntaxin-6. The NO scavenger (4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide failed to affect the initiation or progression of MCTP megalocytosis despite a reduction of 4,5-diaminofluorescein diacetate fluorescence and inhibition of S-nitrosylation of eNOS as assayed using the biotin-switch method. Moreover, the latter assay not only revealed constitutive S-nitrosylation of NSF, eNOS, cav-1, and clathrin heavy chain (CHC) in PAECs but also a dramatic 70-95% decrease in the S-nitrosylation of NSF, eNOS, cav-1, and CHC after MCTP. These data point to depletion of NSF from Golgi membranes as a mechanism for Golgi blockade after MCTP and to denitrosylation of vasorelevant proteins as critical to the development of endothelial cell megalocytosis.
Figures
Fig. 1.
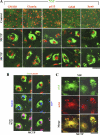
Confocal immunofluorescence imaging showing separation of _N_-ethylmaleimide-sensitive factor (NSF) from Golgi tethers and soluble NSF-attachment protein receptors (SNAREs) in monocrotaline pyrrole (MCTP)-induced megalocytosis of endothelial cells. Pulmonary arterial endothelial cells (PAECs) in 6-well plates were treated with MCTP, and megalocytosis was allowed to develop for 4 days. Control and MCTP-treated cultures were then fixed using the cold paraformaldehyde-Triton X-100 fixation protocol. Two-color immunofluorescence analyses were carried out using goat polyclonal antibodies (PAbs) to NSF in combination with either of murine monoclonal antibodies (MAbs) to Golgi matrix (GM)130, Golgi snare (GS)28, or syntaxin (Syn)-6 (A) or rabbit PAbs to giantin or p115 (A and B) as indicated. As a further control, a murine MAb to NSF was used in combination with the goat PAb to the NSF in C. A, arrowheads: cells with a diffuse NSF pattern after MCTP. *Cells with punctate NSF. Scale bars, 50 μm. eNOS, endothelial nitric oxide synthase.
Fig. 2.
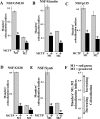
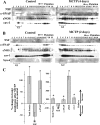
Quantitative image analyses of changes in colocalization of NSF with Golgi tethers and SNAREs in MCTP-induced megalocytosis of endothelial cells. Multiple 246 μm × 246 μm frames (n = 10–15/culture) were collected from each of the experiments in Fig. 1, and quantitative colocalization analyses were carried out using the just another colocalization plug-in (JACoP) of National Institutes of Health ImageJ. Colocalization between NSF and each of the Golgi tethers or SNAREs investigated was assessed using the Manders' colocalization coefficients M1 and M2, which, respectively, represent the overlap between red pixels compared with green pixels as the denominator and vice versa. For each Golgi marker, n > 100 cells in both control and MCTP-treated groups were quantitated. A–E summarize data derived from these 2-way comparisons with M1 and M2 expressed as means ± SE. F diagrams that Manders' coefficients range from 0 to 1 with a higher numerical value indicating increasing colocalization. *P < 0.00001, using the Student's _t_-test.
Fig. 3.
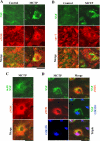
Confocal immunofluorescence imaging showing separation of NSF from Golgi cargo proteins eNOS and caveolin (cav)-1 in MCTP-induced megalocytosis of endothelial cells. PAECs in 6-well plates were treated with MCTP, and megalocytosis was allowed to develop for 4 days. Control and MCTP-treated cultures were then fixed using the cold paraformaldehyde-Triton X-100 fixation protocol. Two-color immunofluorescence analyses were carried out using goat PAb to NSF in combination with either of rabbit PAbs to eNOS (A) or cav-1 (B) or with a murine MAb to NSF along with a rabbit PAb to eNOS (C). In D, a murine MAb to the Golgi tether GM130 was used for immunofluorescence in combination with the goat PAb to NSF and the rabbit PAb to eNOS. Scale bars, 50 μm.
Fig. 4.
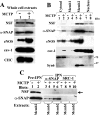
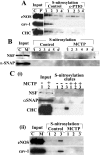
Cellular levels of NSF and its capacity to associate with α-soluble NSF-attachment protein (SNAP) are maintained in MCTP-induced megalocytosis of endothelial cells. PAECs in 10-cm plates were treated with MCTP (8 cultures) or with vehicle alone (4 cultures), and megalocytosis was allowed to develop over 4 days. A: whole-cell extracts were prepared using the lysis buffer in the _S_-nitrosylation kit (Cayman Chemicals) and assayed for total protein using the Bradford assay, and protein-matched aliquots were Western blotted for respective proteins. Quantitation of the blot shown in A revealed that the total cellular content of NSF was 114% after MCTP compared with that in the control cells. B: cytoplasmic extracts were prepared by sequentially harvesting each group in isotonic buffer containing 50 μg/ml of digitonin (cytosol), 0.5% Brij-58 [membrane 1 (Memb1)], and 0.5% deoxycholate/1% Tween 40 [membrane 2 (Memb2)], leaving a pellet fraction (nucleus). A composite of the Western blot analyses performed using protein-matched aliquots of extracts derived from control and MCTP-treated cells is shown. Quantitation of the blot shown in B revealed that the total cellular content of NSF was 101% after MCTP compared with that in the control cells. In C, protein-matched aliquots of respective membrane fractions derived from control and MCTP-treated samples were immunopanned using PAbs to α-SNAP and steroid receptor coactivator (SRC)-1 in the presence of 0.1% SDS. Arrow indicates cross-immunopanning (IPN) of NSF by anti-α-SNAP PAb. CHC, clathrin heavy chain.
Fig. 5.
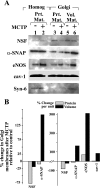
Selective depletion of NSF from purified Golgi membranes derived from endothelial cells with MCTP-induced megalocytosis. PAECs in 10-cm plates were treated with MCTP (8 cultures) or vehicle (4 cultures), and megalocytosis was allowed to develop for 4 (A) or 2 (B) days. Golgi membranes were purified from protein-matched aliquots of the respective clarified cell homogenates by the flotation up a discontinuous sucrose gradient using the method of Xu and Shields (Ref. 60). Five hundred microliter fractions were collected sequentially from the top, and 250 μl of each fraction were used for trichloroacetic acid (TCA) precipitation. Western blot analyses were carried out using the TCA-precipitated aliquots of each fraction and MAbs to NSF, α-SNAP, or Syn-6 or rabbit PAbs to eNOS or cav-1. C: quantitation of the change in association of total protein or various specific proteins with the Golgi fractions (fractions 3, 4, and 5 in each gradient) at the 0.8/1.2 M sucrose interface (pooled as G1 and G2) from 3 independent experiments expressed in terms of the values in controls (as percent change means ± SE relative to that in controls). *P < 0.01.
Fig. 6.
Selective depletion of NSF per-unit Golgi protein in MCTP-induced megalocytosis of endothelial cells. The respective Golgi fractions from the experiment depicted in Fig. 5_B_ (fractions 3, 4, and 5 from both the control and MCTP gradients) were pooled into the G1 and G2 pools. Aliquots were used for determination of total protein in the respective G1 and G2 pools. Western blotting was carried out following TCA precipitation in 2 ways: either based upon protein-matched (Prt Mat) aliquots (250 μl for G1 and 88 μl for G2) or Golgi compartment volume-matched (Vol Mat) aliquots (250 μl each of G1 and G2) using respective antibodies. For comparison, protein-matched aliquots of the starting cell homogenate (Homog) were included in lanes 1 and 2. B: quantitation of the Western blot data shown in A.
Fig. 7.
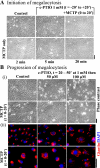
(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) blocked neither the initiation nor progression of MCTP-induced megalocytosis in endothelial cells. In A, PAECs in 6-well plates were left untreated; treated with MCTP for 2, 5, or 20 min and then replenished with MCTP-free medium; treated with 1 mM c-PTIO alone for 40 min and then replenished with c-PTIO-free medium; or treated with 1 mM c-PTIO for 20 min followed by MCTP and 1 mM c-PTIO for an additional 20 min and then replenished with MCTP and c-PTIO-free medium as indicated. Phase contrast pictures were taken 2 days after the beginning of the experiment. Scale bar, 50 μM. In B, PAECs in 6-well plates were first treated with MCTP for 20 min, washed, and then treated with 1 mM c-PTIO for 30 min and maintained in medium containing 50 or 100 μM c-PTIO for 2 days. Respective controls included cultures, which received MCTP alone or c-PTIO alone or neither as indicated. Phase contrast images were collected 2 days after the beginning of the experiment. Cultures were then fixed using the cold paraformaldehyde Triton X-100 protocol, and immunofluorescence analyses were carried out using a rabbit PAb to giantin and 4',6-diamidino-2-phenylindole (DAPI). Scale bars, 50 μm.
Fig. 8.
Constitutive _S_-nitrosylation of eNOS, NSF, CHC, and cav-1 in endothelial cells and hypo-_S_-nitrosylation after c-PTIO or MCTP. PAEC cultures in 10-cm plates were left untreated or treated with 100 μM c-PTIO for 2 days (4 cultures/group; A) or were treated with MCTP (8 cultures) or vehicle (4 cultures), and megalocytosis was allowed to develop for 4 days (B and C). _S_-nitrosylated proteins were detected using the biotin-switch assay (Refs. and 23) in which S_-nitrosylated proteins in protein-matched aliquots of the whole-cell extracts are first selectively biotinylated, then purified using a NeutrAvidin spin column, and the biotinylation reversed by elution in buffer containing 100 mM β-mercaptoethanol (Refs. and 23) in sequential 100-μl elution steps. Western blot analyses of the respective eluate fractions were carried out using 50-μl aliquots in A, B, and C(i) and 35 μl in C(ii) and respective antibodies as indicated; input lanes containing protein-matched aliquots of the respective whole-cell extracts were included within the same blot [for comparison the input lanes in B (which are the same as in Fig. 4_A) and in C are illustrated next to the eluate lanes as an uncut set of continuous lanes in the same enhanced chemiluminescence exposure]. Quantitation of _S_-nitrosylated pools of the respective proteins detected in the Western blots was carried out by summing across the eluted fractions. In A, c-PTIO reduced _S-_nitrosylation of eNOS by 70%, of cav-1 by 99%, and of CHC by 89%. In B, MCTP reduced _S_-nitrosylation of NSF by >95%. In C, MCTP reduced _S-_nitrosylation of NSF by 81%, of CHC by 97%, of eNOS by 95%, and of cav-1 by 94%. C, control; P, c-PTIO; M, MCTP.
Similar articles
- Golgi, trafficking, and mitosis dysfunctions in pulmonary arterial endothelial cells exposed to monocrotaline pyrrole and NO scavenging.
Lee J, Reich R, Xu F, Sehgal PB. Lee J, et al. Am J Physiol Lung Cell Mol Physiol. 2009 Oct;297(4):L715-28. doi: 10.1152/ajplung.00086.2009. Epub 2009 Jul 31. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19648287 Free PMC article. - Dysfunction of Golgi tethers, SNAREs, and SNAPs in monocrotaline-induced pulmonary hypertension.
Sehgal PB, Mukhopadhyay S, Xu F, Patel K, Shah M. Sehgal PB, et al. Am J Physiol Lung Cell Mol Physiol. 2007 Jun;292(6):L1526-42. doi: 10.1152/ajplung.00463.2006. Epub 2007 Mar 2. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17337506 - Aberrant cytoplasmic sequestration of eNOS in endothelial cells after monocrotaline, hypoxia, and senescence: live-cell caveolar and cytoplasmic NO imaging.
Mukhopadhyay S, Xu F, Sehgal PB. Mukhopadhyay S, et al. Am J Physiol Heart Circ Physiol. 2007 Mar;292(3):H1373-89. doi: 10.1152/ajpheart.00990.2006. Epub 2006 Oct 27. Am J Physiol Heart Circ Physiol. 2007. PMID: 17071725 - Cellular functions of NSF: not just SNAPs and SNAREs.
Zhao C, Slevin JT, Whiteheart SW. Zhao C, et al. FEBS Lett. 2007 May 22;581(11):2140-9. doi: 10.1016/j.febslet.2007.03.032. Epub 2007 Mar 21. FEBS Lett. 2007. PMID: 17397838 Free PMC article. Review. - SNAREs and membrane fusion in the Golgi apparatus.
Nichols BJ, Pelham HR. Nichols BJ, et al. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):9-31. doi: 10.1016/s0167-4889(98)00044-5. Biochim Biophys Acta. 1998. PMID: 9714710 Review.
Cited by
- Evidence for Multiple Origins of De Novo Formed Vascular Smooth Muscle Cells in Pulmonary Hypertension: Challenging the Dominant Model of Pre-Existing Smooth Muscle Expansion.
Chu X, Ahmadvand N, Zhang JS, Seeger W, Bellusci S, El Agha E. Chu X, et al. Int J Environ Res Public Health. 2021 Aug 14;18(16):8584. doi: 10.3390/ijerph18168584. Int J Environ Res Public Health. 2021. PMID: 34444333 Free PMC article. Review. - The Ever-Expanding Influence of the Endothelial Nitric Oxide Synthase.
Rafea R, Siragusa M, Fleming I. Rafea R, et al. Basic Clin Pharmacol Toxicol. 2025 May;136(5):e70029. doi: 10.1111/bcpt.70029. Basic Clin Pharmacol Toxicol. 2025. PMID: 40150952 Free PMC article. Review. - Golgi, trafficking, and mitosis dysfunctions in pulmonary arterial endothelial cells exposed to monocrotaline pyrrole and NO scavenging.
Lee J, Reich R, Xu F, Sehgal PB. Lee J, et al. Am J Physiol Lung Cell Mol Physiol. 2009 Oct;297(4):L715-28. doi: 10.1152/ajplung.00086.2009. Epub 2009 Jul 31. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19648287 Free PMC article. - Nitric Oxide Interacts with Caveolin-1 to Facilitate Autophagy-Lysosome-Mediated Claudin-5 Degradation in Oxygen-Glucose Deprivation-Treated Endothelial Cells.
Liu J, Weaver J, Jin X, Zhang Y, Xu J, Liu KJ, Li W, Liu W. Liu J, et al. Mol Neurobiol. 2016 Nov;53(9):5935-5947. doi: 10.1007/s12035-015-9504-8. Epub 2015 Oct 29. Mol Neurobiol. 2016. PMID: 26515186 Free PMC article. - Golgi dysfunction is a common feature in idiopathic human pulmonary hypertension and vascular lesions in SHIV-nef-infected macaques.
Sehgal PB, Mukhopadhyay S, Patel K, Xu F, Almodóvar S, Tuder RM, Flores SC. Sehgal PB, et al. Am J Physiol Lung Cell Mol Physiol. 2009 Oct;297(4):L729-37. doi: 10.1152/ajplung.00087.2009. Epub 2009 Jul 31. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19648286 Free PMC article.
References
- Baur T, Ramadan K, Schlundt A, Kartenbeck J, Meyer HH. NSF- and SNARE-mediated membrane fusion is required for nuclear envelope formation and completion of nuclear pore complex assembly in Xenopus laevis egg extracts. J Cell Sci 120: 2895–2903, 2007. - PubMed
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232, 2006. - PubMed
- Bonifacino JS, Glick BS. The mechanism of vesicle budding and fusion. Cell 116: 153–166, 2004. - PubMed
- Cheretien A, Piront N, Delaive E, Demazy C, Ninane N, Toussaint O. Increased abundance of cytoplasmic and nuclear caveolin 1 in human diploid fibroblasts in H2O2-induced premature senescence and interplay with p38αMAPK. FEBS Lett 582: 1685–1692, 2008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases