Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke - PubMed (original) (raw)
Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke
Wenlan Liu et al. J Neurochem. 2008 Dec.
Abstract
Matrix metalloproteinase-9 (MMP-9) and NADPH oxidase contribute to blood-brain barrier (BBB) disruption after ischemic stroke. We have previously shown that normobaric hyperoxia (NBO) treatment reduces MMP-9 and oxygen free radical generation in ischemic brain. In this study, we tested the hypothesis that NBO protects the BBB through inhibiting NADPH oxidase-mediated MMP-9 induction in transient focal cerebral ischemia. Male Sprague-Dawley rats (n = 69) were given NBO (95% O2) or normoxia (21% O2) during 90-min filament occlusion of the middle cerebral artery. Cerebral microvessels were isolated for analyzing MMP-9 and NADPH oxidase. BBB damage was non-invasively quantified with magnetic resonance imaging. In normoxic rats, both NADPH oxidase catalytic subunit gp91(phox) and MMP-9 expression were up-regulated in ischemic hemispheric microvessels after 90-min middle cerebral artery occlusion with 22.5 h reperfusion. Inhibition of NADPH oxidase with apocynin reduced the MMP-9 increase, indicating a causal link between NADPH oxidase-derived superoxide and MMP-9 induction. NBO treatment inhibited gp91(phox) expression, NADPH oxidase activity, and MMP-9 induction, which led to significantly less BBB damage and brain edema in the ischemic brain. These results suggest that gp91(phox) containing NADPH oxidase plays an important role in MMP-9 induction in ischemic BBB microvasculature, and that NBO treatment may attenuate MMP-9 induction and brain edema through inhibiting NADPH oxidase after transient cerebral ischemia.
Figures
Figure 1
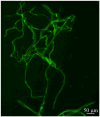
Representative fluorescent micrograph of cerebral microvessl preparation after immunostaining of tight junction protein claudin-5.
Figure 2
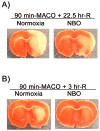
TTC staining verified successful MCAO in normoxic and NBO-treated rats. Typical TTC staining of the 1-mm thick brain slice 6 mm away from the tip of frontal lobe after 90-min MCAO with 22.5 (A) or 3 (B) hrs of reperfusion. NBO was delivered during 90-min ischemia.
Figure 3
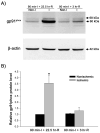
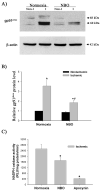
Gp91phox protein expression in cerebral microvessels after 90-min MCAO with 3- or 22.5-hr reperfusion. Cerebral microvessel lysates (50 μg) were analyzed for gp91phox protein by western blot. As a loading control, the blots were stripped and re-blotted with β-actin antibody. A) Representative blots of gp91phox and corresponding β-actin are shown. Non-I: nonischemic hemispheric microvessels; I: ischemic hemispheric microvessels. B) The relative quantity of protein was calculated after normalization to β-actin. The gp91phox protein expression was significantly increased in the ischemic hemispheric microvessels in those rats reperfused for 22.5 hrs (n = 6, *p < 0.05 versus nonischemic hemispheric microvessels). Only a slight increase of gp91phox was observed for those rats reperfused for 3 hrs (n =5, _P_ > 0.05). Data are expressed as mean ± SEM.
Figure 4
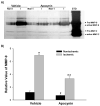
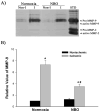
Effect of apocynin on MMP-9 induction in ischemic cerebral microvessels after 90-min MCAO with 22.5-hr reperfusion. Apocynin (30 mg/kg body weight) or vehicle (0.15 ml/kg body weight) was intraperitoneally injected to rats 1 hr before the onset of MCAO. Cerebral microvessel lysates (50 μg) were analyzed for MMP-9 expression with gelatin zymography. A) Representative gelatin zymogram showing MMP-9 expression in the nonischemic (Non-I) and ischemic (I) hemispheric cerebral microvessels. MMP-9 was clearly visualized on the gelatin zymogram, while MMP-2 was barely visible on the gel. STD is a mixture of standard MMP-2 and MMP-9. B) The relative band intensity of MMP-9 was quantified. A significant increase was observed for MMP-9 expression in the ischemic hemispheric microvessels in both vehicle- and apocynin-treated rats (*p < 0.05 versus nonischemic hemispheric microvessels). Apocynin significantly reduced MMP-9 expression in both nonischemic and ischemic hemispheric microvessels (#p < 0.05 versus vehicle-treated rats). Data are expressed as mean ± SEM, n = 3 in the vehicle-treated group, n = 6 in apocynin-treated group.
Figure 5
The effect of NBO on gp91phox-containing NADPH oxidase in ischemic hemispheric microvessels after 90-min MCAO with 22.5-hr reperfusion. A) Representative blots of gp91phoxand corresponding β-actin are shown. Cerebral microvessel lysates (50 μg protein) were analyzed for gp91phox protein by western blot. As a loading control, the blots were stripped and reblotted with β-actin antibody. Non-I: nonischemic hemispheric microvessels; I: ischemic hemispheric microvessels. B) The relative quantity of protein was calculated after normalization to β-actin. Ischemia and reperfusion significantly increased gp91phox protein expression in the ischemic hemispheric microvessels (*p < 0.05 versus nonischemic hemispheric microvessels), which was significantly inhibited by NBO treatment (*p < 0.05 versus normoxic rats). Data are expressed as mean ±SEM, n = 6 in each group. C) NADPH oxidase activity in ischemic hemispheric microvessels was assayed using lucigenin-enhanced chemiluminescence. NBO orapocynin treatment significantly reduced NADPH oxidase activity (*p < 0.05). Data are expressed as mean ±SEM, n = 6 in each of normoxic and NBO-treated group, n = 5 in apocynin-treated group.
Figure 6
Effect of NBO on MMP-9 induction in ischemic cerebral microvessels after 90-min MCAO with 22.5-hr reperfusion. Cerebral microvessel lysates (50 μg) were analyzed for MMP-9 expression with gelatin zymography. A) Representative gelatin zymogram showing MMP-9 expression in the nonischemic (Non-I) and ischemic (I) hemispheric cerebral microvessels. STD is a mixture of standard MMP-2 and MMP-9. B) The relative band intensity of MMP-9 was quantified. A significant increase was observed for MMP-9 expression in the ischemic hemispheric microvessels in both normoxic and NBO-treated rats (*P < 0.05 versus nonischemic hemispheric microvessels). NBO significantly reduced MMP-9 expression in the ischemic hemispheric microvessels (#P < 0.05 versus vehicle-treated rats). Data are expressed as mean ±SEM, n = 6 in each group.
Figure 7
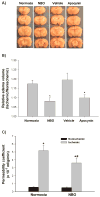
Effect of NBO on brain edema formation and BBB permeability after 90-min MCAO with 22.5-hr reperfusion. A) Representative brain sections of the normoxic, NBO-, vehicle- and apocynin-treated rats. Significant right (ischemic) hemispheric enlargement is seen in rats of all groups. B) Quantitative image analysis showed a significant reduction of hemispheric enlargement in the NBO- or apocynin-treated rats when compared to normoxic rats. Data are expressed as mean ±SEM, n = 4 in the vehicle-treated group, n = 7 in each of other three groups. *P < 0.05 versus normoxic rats. C) Effect of NBO on BBB permeability coefficient assessed by MRI based technique. A significant increase was observed for BBB permeability coefficient in the ischemic hemisphere of both normoxic and NBO-treated rats (*P < 0.05 versus nonischemic hemisphere). NBO significantly reduced BBB permeability in the ischemic hemisphere (#P < 0.05 versus normoxic rats). Data are expressed as mean ± SEM, n = 6 in each group.
Similar articles
- NADPH oxidase mediates the expression of MMP-9 in cerebral tissue after ischemia-reperfusion damage.
Tang X, Zhong W, Tu Q, Ding B. Tang X, et al. Neurol Res. 2014 Feb;36(2):118-25. doi: 10.1179/1743132813Y.0000000266. Epub 2013 Dec 6. Neurol Res. 2014. PMID: 24131725 - Inhibition of gp91(phox) contributes towards normobaric hyperoxia afforded neuroprotection in focal cerebral ischemia.
Tang X, Liu KJ, Ramu J, Chen Q, Li T, Liu W. Tang X, et al. Brain Res. 2010 Aug 12;1348:174-80. doi: 10.1016/j.brainres.2010.05.082. Epub 2010 Jun 11. Brain Res. 2010. PMID: 20547141 Free PMC article. - Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia.
Liu W, Hendren J, Qin XJ, Shen J, Liu KJ. Liu W, et al. J Neurochem. 2009 Feb;108(3):811-20. doi: 10.1111/j.1471-4159.2008.05821.x. J Neurochem. 2009. PMID: 19187098 Free PMC article. - The Alzheimer's Disease Brain, Its Microvasculature, and NADPH Oxidase.
Mamelak M. Mamelak M. J Alzheimers Dis. 2024;99(s1):S109-S118. doi: 10.3233/JAD-230415. J Alzheimers Dis. 2024. PMID: 37599534 Review. - NADPH oxidases as therapeutic targets in ischemic stroke.
Kahles T, Brandes RP. Kahles T, et al. Cell Mol Life Sci. 2012 Jul;69(14):2345-63. doi: 10.1007/s00018-012-1011-8. Epub 2012 May 23. Cell Mol Life Sci. 2012. PMID: 22618244 Free PMC article. Review.
Cited by
- Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions.
Krueger M, Härtig W, Reichenbach A, Bechmann I, Michalski D. Krueger M, et al. PLoS One. 2013;8(2):e56419. doi: 10.1371/journal.pone.0056419. Epub 2013 Feb 26. PLoS One. 2013. PMID: 23468865 Free PMC article. - Protective effect of ginsenoside Rb1 on integrity of blood-brain barrier following cerebral ischemia.
Chen W, Guo Y, Yang W, Zheng P, Zeng J, Tong W. Chen W, et al. Exp Brain Res. 2015 Oct;233(10):2823-31. doi: 10.1007/s00221-015-4352-3. Epub 2015 Jun 13. Exp Brain Res. 2015. PMID: 26070903 - Pinocembrin Protects Blood-Brain Barrier Function and Expands the Therapeutic Time Window for Tissue-Type Plasminogen Activator Treatment in a Rat Thromboembolic Stroke Model.
Ma Y, Li L, Kong L, Zhu Z, Zhang W, Song J, Chang J, Du G. Ma Y, et al. Biomed Res Int. 2018 Apr 22;2018:8943210. doi: 10.1155/2018/8943210. eCollection 2018. Biomed Res Int. 2018. PMID: 29850586 Free PMC article. - Cocktail treatment, a promising strategy to treat acute cerebral ischemic stroke?
Liang LJ, Yang JM, Jin XC. Liang LJ, et al. Med Gas Res. 2016 Apr 4;6(1):33-38. doi: 10.4103/2045-9912.179343. eCollection 2016 Mar. Med Gas Res. 2016. PMID: 27826421 Free PMC article. Review. - Melatonin alleviates lipopolysaccharide-compromised integrity of blood-brain barrier through activating AMP-activated protein kinase in old mice.
Wang X, Xue GX, Liu WC, Shu H, Wang M, Sun Y, Liu X, Sun YE, Liu CF, Liu J, Liu W, Jin X. Wang X, et al. Aging Cell. 2017 Apr;16(2):414-421. doi: 10.1111/acel.12572. Epub 2017 Feb 3. Aging Cell. 2017. PMID: 28156052 Free PMC article.
References
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:452–457. - PubMed
- Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. - PubMed
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. - PubMed
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR015636/RR/NCRR NIH HHS/United States
- P20 RR015636-08/RR/NCRR NIH HHS/United States
- R01 AG031725/AG/NIA NIH HHS/United States
- P20 RR15636/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous