The p38 mitogen-activated protein kinase augments nucleotide excision repair by mediating DDB2 degradation and chromatin relaxation - PubMed (original) (raw)
The p38 mitogen-activated protein kinase augments nucleotide excision repair by mediating DDB2 degradation and chromatin relaxation
Qun Zhao et al. J Biol Chem. 2008.
Abstract
The p38 MAPK is a family of serine/threonine protein kinases that play important roles in cellular responses to external stress signals, e.g. UV irradiation. To assess the role of p38 MAPK pathway in nucleotide excision repair (NER), the most versatile DNA repair pathway, we determined the efficiency of NER in cells treated with p38 MAPK inhibitor SB203580 and found that p38 MAPK is required for the prompt repair of UV-induced DNA damage CPD. We further investigated the possible mechanism through which p38 MAPK regulates NER and found that p38 MAPK mediates UV-induced histone H3 acetylation and chromatin relaxation. Moreover, p38 MAPK also regulates UV-induced DDB2 ubiquitylation and degradation via phosphorylation of the target protein. Finally, our results showed that p38 MAPK is required for the recruitment of NER factors XPC and TFIIH to UV-induced DNA damage sites. We conclude that p38 MAPK regulates chromatin remodeling as well as DDB2 degradation for facilitating NER factor assembly.
Figures
FIGURE 1.
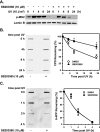
p38 MAPK inhibitor SB203580 inhibits the activation of p38 MAPK but not JNK, ERK, or Akt in response to UV irradiation. A, chemical structure of SB203580. B and C, inhibition of p38 MAPK kinase activity by different doses of SB203580. OSU-2 cells (B) or HeLa-DDB2 cells (C) were treated with SB203580 for 30 min at indicated concentrations and UV-irradiated at 20 J/m2. The cells were cultured in the same medium for 1 h, and the cell lysates were analyzed by Western blot analysis using anti-phospho-MK2 antibody. Lamin B was detected to serve as an internal control. D–F, inhibition of p38 MAPK, JNK, ERK, and Akt activation by SB203580. OSU-2 cells (D and_E_) or HeLa-DDB2 cells (F) were treated with DMSO or SB203580 (10 μ
m
) for 30 min and UV-irradiated at indicated doses. The cells were allowed to repair for indicated time periods (D) or 30 min (E) or 2 h (F), and the whole cell lysates were analyzed by Western blotting. Whole cell lysates of CP70 cells (lanes marked_C_), having overexpressed Akt, were used as a positive control of Akt phosphorylation. Lamin B, from the same gels, was used as a loading control.
FIGURE 2.
p38 MAPK is required for efficient removal of CPD. A, inhibition of p38 MAPK activation by SB203580. OSU-2 cells were pretreated with DMSO or SB203580 (10 μ
m
) for 30 min. The cells were then UV-irradiated at 10 J/m2 and further cultured in the same medium for indicated time periods. The cell lysates were analyzed for phospho-MK2 levels using Western blotting. Lamin B was detected as an internal control.B and C, effect of p38 MAPK on removal of CPD or 6-4PP. OSU-2 cells were cultured in serum-free medium for 48 h before the start of different treatments. The cells were treated with SB203580 (10 μ
m
) or DMSO for 30 min and then UV-irradiated at 10 J/m2 and allowed to repair for indicated times in the same medium. Identical amounts of genomic DNA were subjected to ISB analysis, and the amounts of CPD or 6-4PP at different times were detected with anti-CPD or anti-6-4PP antibody, respectively. The intensity of each band was quantified, and the relative CPD or 6-4PP remaining in different samples were calculated based on the initial CPD or 6-4PP formation. The data presented in the graph on the right indicate the means ± S.E. of the relative CPD or 6-4PP remaining from three independent experiments. *, significant difference between DMSO- and SB203580-treated cells, p < 0.05.
FIGURE 3.
p38 MAPK is involved in UV-induced chromatin relaxation. A, cell cycle distribution. OSU-2 cells grown in complete medium (top panel) and serum free medium (bottom panel) were analyzed by flow cytometry for cell cycle distribution. B, MNase sensitivity in UV-irradiated cells. OSU-2 cells were serum-starved for 48 h and then treated with DMSO or SB203580 for 30 min. The cells were UV-irradiated at 200 J/m2 and cultured for additional 30 min before nuclei isolation. MNase digestion and agarose gel electrophoresis was conducted to determine the extent of chromatin relaxation. C, histone acetylation levels in response to UV irradiation. Growth-arrested OSU-2 cells were pretreated with DMSO or SB203580, UV-irradiated at 20 J/m2, and allowed to repair for indicated time periods. The whole cell lysates were subjected to Western blotting, and acetylated histone H3 was detected with anti-AcH3 (K9, 14), anti-AcH3 (K14), or anti-AcH3 (K18). Actin in the same gels was detected as loading control. D, histone H3 phosphorylation upon UV irradiation. Growth-arrested OSU-2 cells were treated as described in C. Anti-p-H3 (Ser10) antibody was used to detect the phosphorylated H3 (Ser10) level in the whole cell lysates.
FIGURE 4.
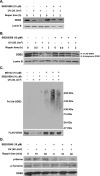
p38 MAPK mediates UV-induced DDB2 ubiquitylation and degradation. A and B, OSU-2 (A) or HeLa-DDB2 (B) cells were treated with DMSO or SB203580 for 30 min, UV-irradiated at 20 J/m2, and further incubated for indicated times. The whole cell extracts were generated by boiling of the cells in SDS lysis buffer and then subjected to Western blotting with anti-DDB2 and anti-Lamin B antibodies.C, DDB2 ubiquitylation upon UV treatment. HeLa-DDB2 cells were pretreated with MG132 together with SB203580 for 30 min and UV-irradiated at 40 J/m2. The cells were cultured in the same medium for 2 h, and the cell lysates were immunoprecipitated with anti-FLAG M2 affinity gel. The immunoprecipitates were subjected to Western blot analysis with anti-ubiquitin or anti-DDB2 antibody. D, DDB2 phosphorylation by p38 MAPK. HeLa-DDB2 cells were pretreated with DMSO or SB203580 for 30 min prior to UV irradiation at 20 J/m2. The cells were kept in the same medium for the indicated times, and the whole cell lysates were prepared. FLAG-tagged DDB2 was immunoprecipitated with anti-FLAG M2 affinity gel and analyzed by Western blotting with anti-phospho-serine, anti-phospho-tyrosine, or anti-DDB2 antibody.
FIGURE 5.
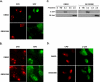
p38 MAPK affects the recruitment of XPC and XPB to CPD foci. A and B, recruitment of XPC and XPB to CPD. OSU-2 cells were grown on coverslips and pretreated with DMSO or SB203580 for 30 min prior to UV irradiation (100 J/m2) through a 5-μm isopore polycarbonate filter. The cells were incubated in medium for 15 min and then fixed with 2% paraformaldehyde. The cells were double-stained with mouse anti-CPD antibody and rabbit anti-XPC antibody or with mouse anti-CPD antibody and rabbit anti-XPB antibody. C, binding of DDB2 to UV-damaged chromatin. OSU-2 cells were treated with DMSO or SB203580 (10 μ
m
) for 30 min and then UV-irradiated at 20 J/m2. The cells were incubated in the same medium for another 30 min and subjected to cellular protein fractionation. Five fractions as indicated according to the resistance to detergent and salt were obtained. S, cytoplasmic soluble protein; TW, nucleoplasmic soluble proteins; 0.3, proteins binding to chromatin loosely; 0.5, proteins binding to chromatin with intermediate affinity; 2.0, proteins binding to chromatin tightly. Each protein fraction, corresponding to an equivalent cell number, was loaded for SDS-PAGE and analyzed by immunoblotting with anti-DDB2 antibody. D, recruitment of p38 MAPK to the damage site. The cells were treated as described in A and B and double-stained with mouse anti-CPD antibody and rabbit anti-phospho-p38 antibody.
FIGURE 6.
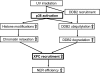
Proposed model for positive modulation of NER by p38 MAPK activation following UV irradiation of human cells.
Similar articles
- Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway.
Kovarik P, Stoiber D, Eyers PA, Menghini R, Neininger A, Gaestel M, Cohen P, Decker T. Kovarik P, et al. Proc Natl Acad Sci U S A. 1999 Nov 23;96(24):13956-61. doi: 10.1073/pnas.96.24.13956. Proc Natl Acad Sci U S A. 1999. PMID: 10570180 Free PMC article. - p38 MAPK- and Akt-mediated p300 phosphorylation regulates its degradation to facilitate nucleotide excision repair.
Wang QE, Han C, Zhao R, Wani G, Zhu Q, Gong L, Battu A, Racoma I, Sharma N, Wani AA. Wang QE, et al. Nucleic Acids Res. 2013 Feb 1;41(3):1722-33. doi: 10.1093/nar/gks1312. Epub 2012 Dec 28. Nucleic Acids Res. 2013. PMID: 23275565 Free PMC article. - The cullin 4B-based UV-damaged DNA-binding protein ligase binds to UV-damaged chromatin and ubiquitinates histone H2A.
Guerrero-Santoro J, Kapetanaki MG, Hsieh CL, Gorbachinsky I, Levine AS, Rapić-Otrin V. Guerrero-Santoro J, et al. Cancer Res. 2008 Jul 1;68(13):5014-22. doi: 10.1158/0008-5472.CAN-07-6162. Cancer Res. 2008. PMID: 18593899 - Open, repair and close again: chromatin dynamics and the response to UV-induced DNA damage.
Palomera-Sanchez Z, Zurita M. Palomera-Sanchez Z, et al. DNA Repair (Amst). 2011 Feb 7;10(2):119-25. doi: 10.1016/j.dnarep.2010.10.010. Epub 2010 Dec 3. DNA Repair (Amst). 2011. PMID: 21130713 Review. - Mechanism and regulation of DNA damage recognition in mammalian nucleotide excision repair.
Sugasawa K. Sugasawa K. Enzymes. 2019;45:99-138. doi: 10.1016/bs.enz.2019.06.004. Epub 2019 Jul 8. Enzymes. 2019. PMID: 31627884 Review.
Cited by
- PTEN positively regulates UVB-induced DNA damage repair.
Ming M, Feng L, Shea CR, Soltani K, Zhao B, Han W, Smart RC, Trempus CS, He YY. Ming M, et al. Cancer Res. 2011 Aug 1;71(15):5287-95. doi: 10.1158/0008-5472.CAN-10-4614. Epub 2011 Jul 19. Cancer Res. 2011. PMID: 21771908 Free PMC article. - Human SNF5/INI1, a component of the human SWI/SNF chromatin remodeling complex, promotes nucleotide excision repair by influencing ATM recruitment and downstream H2AX phosphorylation.
Ray A, Mir SN, Wani G, Zhao Q, Battu A, Zhu Q, Wang QE, Wani AA. Ray A, et al. Mol Cell Biol. 2009 Dec;29(23):6206-19. doi: 10.1128/MCB.00503-09. Epub 2009 Oct 5. Mol Cell Biol. 2009. PMID: 19805520 Free PMC article. - Stem cell protein Piwil2 modulates chromatin modifications upon cisplatin treatment.
Wang QE, Han C, Milum K, Wani AA. Wang QE, et al. Mutat Res. 2011 Mar 15;708(1-2):59-68. doi: 10.1016/j.mrfmmm.2011.02.001. Epub 2011 Feb 15. Mutat Res. 2011. PMID: 21310163 Free PMC article. - The nucleotide excision repair protein XPC is essential for bulky DNA adducts to promote interleukin-6 expression via the activation of p38-SAPK.
Schreck I, Grico N, Hansjosten I, Marquardt C, Bormann S, Seidel A, Kvietkova DL, Pieniazek D, Segerbäck D, Diabaté S, van der Horst GT, Oesch-Bartlomowicz B, Oesch F, Weiss C. Schreck I, et al. Oncogene. 2016 Feb 18;35(7):908-18. doi: 10.1038/onc.2015.145. Epub 2015 May 18. Oncogene. 2016. PMID: 25982271 - The NR4A2 nuclear receptor is recruited to novel nuclear foci in response to UV irradiation and participates in nucleotide excision repair.
Jagirdar K, Yin K, Harrison M, Lim W, Muscat GE, Sturm RA, Smith AG. Jagirdar K, et al. PLoS One. 2013 Nov 6;8(11):e78075. doi: 10.1371/journal.pone.0078075. eCollection 2013. PLoS One. 2013. PMID: 24223135 Free PMC article.
References
- De Laat, W. L., Jaspers, N. G., and Hoeijmakers, J. H. (1999) Genes Dev. 13 768-785 - PubMed
- Hanawalt, P. C. (2002) Oncogene 21 8949-8956 - PubMed
- Hanawalt, P., and Mellon, I. (1994) Curr. Biol. 3 67-69 - PubMed
- Sugasawa, K., Ng, J. M. Y., Masutani, C., Iwai, S., Van der Spek, P., Eker, A., Hanoaka, F., Bootsma, D., and Hoeijmakers, J. H. J. (1998) Mol. Cell 2 223-232 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials