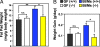Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41 - PubMed (original) (raw)
Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41
Buck S Samuel et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The distal human intestine harbors trillions of microbes that allow us to extract calories from otherwise indigestible dietary polysaccharides. The products of polysaccharide fermentation include short-chain fatty acids that are ligands for Gpr41, a G protein-coupled receptor expressed by a subset of enteroendocrine cells in the gut epithelium. To examine the contribution of Gpr41 to energy balance, we compared Gpr41-/- and Gpr41+/+ mice that were either conventionally-raised with a complete gut microbiota or were reared germ-free and then cocolonized as young adults with two prominent members of the human distal gut microbial community: the saccharolytic bacterium, Bacteroides thetaiotaomicron and the methanogenic archaeon, Methanobrevibacter smithii. Both conventionally-raised and gnotobiotic Gpr41-/- mice colonized with the model fermentative community are significantly leaner and weigh less than their WT (+/+) littermates, despite similar levels of chow consumption. These differences are not evident when germ-free WT and germ-free Gpr41 knockout animals are compared. Functional genomic, biochemical, and physiologic studies of germ-free and cocolonized Gpr41-/- and +/+ littermates disclosed that Gpr41-deficiency is associated with reduced expression of PYY, an enteroendocrine cell-derived hormone that normally inhibits gut motility, increased intestinal transit rate, and reduced harvest of energy (short-chain fatty acids) from the diet. These results reveal that Gpr41 is a regulator of host energy balance through effects that are dependent upon the gut microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Microbiota-mediated increase in adiposity is blunted in Bt/Ms cocolonized gnotobiotic _Gpr41_−/− mice. (A) Weights of both epididymal fat pads in _Gpr41_−/− and +/+ littermates that are GF, or raised GF and then colonized at 4 to 6 weeks of age with Bt and Ms (n, 8–14 males per group; three independent experiments). (B) Weight gain (n, 4–9 mice per group; followed one to two times per week for up to 4 weeks, between 5 and 9 weeks of age in the case of GF animals, and for 4 weeks after gavage in the case of Bt/Ms cocolonized gnotobiotic animals). Mean values ± SEM are plotted. *, P < 0.05, **, P < 0.01. N.S., not significantly different.
Fig. 2.
Microbiota-mediated increase in adiposity is blunted in CONV-R _Gpr41_−/− mice. (A) Epididymal fat-pad weights (values from both fat pads were combined for each animal; n, 12 males per group). (B) Weight gain (n, 6 per group; followed one to two times per week for up to 4 weeks, between the ages of 5 and 9 weeks). (C) Adiposity in CONV-R WT and knockout mice, defined by DEXA (n, 9–13 males per group; 8–10 weeks old). (D and E) Fasting (4 h) serum leptin levels plotted against percentage total body fat (n, 5 per group). *, P < 0.05, **, P < 0.01 (Student's t test).
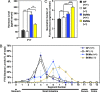
Fig. 3.
Increased rate of intestinal transit in Bt/Ms colonized gnotobiotic _Gpr41_−/− mice. (A) Fasting serum PYY levels in GF and Bt/Ms cocolonized, _Gpr41_−/− and +/+ mice (n = four to eight per group; two independent experiments; all samples assayed in duplicate). (B and C) Gut transit time measured by oral gavage of a fluorescently labeled nonabsorbable substrate (fluorescine isothiocyanate-dextran; 70,000 MW) in GF and cocolonized WT and Gpr41-deficient animals. (B and C) Distribution of fluorescence signal intensity 60 min after gavage along the length of the gut; data are plotted as a fraction of total fluorescence in B. No signal was observed in the ceca or colons in any of the animals. Panel (C) presents the calculated geometric center of the fluorescence (n = 4–8 animals analyzed per group). *, P < 0.05, **, P < 0.01, ***, P < 0.005.
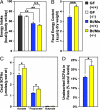
Fig. 4.
Gnotobiotic _Gpr41_−/− mice extract fewer calories from their polysaccharide-rich chow diet and excrete more SCFAs. (A) Dietary energy intake in GF and Bt/Ms cocolonized +/+ and _Gpr41_−/− littermates (calories of chow consumed per day monitored concurrently with body weight, as in Fig. 1). (B) Bomb calorimetry-based assessment of remaining calories in the feces of colonized (Bt/Ms) _Gpr41_−/− and +/+ mice (n = 6–7 per group). (C) GC-MS assay of cecal SCFA levels (n = 5–7 per group; each sample assayed in duplicate). (D) GC-MS study of fecal SFCAs from the same mice assayed in (C). *,P < 0.05, **, P < 0.01, ***, P < 0.005. (Student's t test used for comparisons of two groups.)
Similar articles
- Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut.
Samuel BS, Hansen EE, Manchester JK, Coutinho PM, Henrissat B, Fulton R, Latreille P, Kim K, Wilson RK, Gordon JI. Samuel BS, et al. Proc Natl Acad Sci U S A. 2007 Jun 19;104(25):10643-8. doi: 10.1073/pnas.0704189104. Epub 2007 Jun 11. Proc Natl Acad Sci U S A. 2007. PMID: 17563350 Free PMC article. - A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism.
Samuel BS, Gordon JI. Samuel BS, et al. Proc Natl Acad Sci U S A. 2006 Jun 27;103(26):10011-6. doi: 10.1073/pnas.0602187103. Epub 2006 Jun 16. Proc Natl Acad Sci U S A. 2006. PMID: 16782812 Free PMC article. - Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content.
Bellahcene M, O'Dowd JF, Wargent ET, Zaibi MS, Hislop DC, Ngala RA, Smith DM, Cawthorne MA, Stocker CJ, Arch JR. Bellahcene M, et al. Br J Nutr. 2013 May 28;109(10):1755-64. doi: 10.1017/S0007114512003923. Epub 2012 Oct 31. Br J Nutr. 2013. PMID: 23110765 - Gut microbiota, enteroendocrine functions and metabolism.
Cani PD, Everard A, Duparc T. Cani PD, et al. Curr Opin Pharmacol. 2013 Dec;13(6):935-40. doi: 10.1016/j.coph.2013.09.008. Epub 2013 Sep 24. Curr Opin Pharmacol. 2013. PMID: 24075718 Review. - [Host energy regulation via SCFAs receptors, as dietary nutrition sensors, by gut microbiota].
Kimura I. Kimura I. Yakugaku Zasshi. 2014;134(10):1037-42. doi: 10.1248/yakushi.14-00169. Yakugaku Zasshi. 2014. PMID: 25274213 Review. Japanese.
Cited by
- The effects of gut microbiota on appetite regulation and the underlying mechanisms.
Yu M, Yu B, Chen D. Yu M, et al. Gut Microbes. 2024 Jan-Dec;16(1):2414796. doi: 10.1080/19490976.2024.2414796. Epub 2024 Nov 6. Gut Microbes. 2024. PMID: 39501848 Review. - Asiaticoside improves depressive-like behavior in mice with chronic unpredictable mild stress through modulation of the gut microbiota.
Ren Q, He C, Sun Y, Gao X, Zhou Y, Qin T, Zhang Z, Wang X, Wang J, Wei S, Wang F. Ren Q, et al. Front Pharmacol. 2024 Oct 18;15:1461873. doi: 10.3389/fphar.2024.1461873. eCollection 2024. Front Pharmacol. 2024. PMID: 39494347 Free PMC article. - Association between Turbot (Scophthalmus maximus) Fish Phenotype and the Post-Larval Bacteriome.
Louvado A, Silva DAM, Oliveira V, Castro C, Cleary DFR, Gomes NCM. Louvado A, et al. Microorganisms. 2024 Oct 4;12(10):2014. doi: 10.3390/microorganisms12102014. Microorganisms. 2024. PMID: 39458323 Free PMC article. - Microbiota-derived short chain fatty acids in pediatric health and diseases: from gut development to neuroprotection.
Hsu CY, Khachatryan LG, Younis NK, Mustafa MA, Ahmad N, Athab ZH, Polyanskaya AV, Kasanave EV, Mirzaei R, Karampoor S. Hsu CY, et al. Front Microbiol. 2024 Oct 8;15:1456793. doi: 10.3389/fmicb.2024.1456793. eCollection 2024. Front Microbiol. 2024. PMID: 39439941 Free PMC article. Review. - Cardiovascular Disease May Be Triggered by Gut Microbiota, Microbial Metabolites, Gut Wall Reactions, and Inflammation.
Dicks LMT. Dicks LMT. Int J Mol Sci. 2024 Oct 2;25(19):10634. doi: 10.3390/ijms251910634. Int J Mol Sci. 2024. PMID: 39408963 Free PMC article. Review.
References
- Flint HJ, et al. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nature Rev Microb. 2008;6:121–131. - PubMed
- Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK030292/DK/NIDDK NIH HHS/United States
- R01 DK070977/DK/NIDDK NIH HHS/United States
- P30 DK056341-08/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P30 DK056341-07/DK/NIDDK NIH HHS/United States
- DK30292/DK/NIDDK NIH HHS/United States
- Howard Hughes Medical Institute/United States
- DK70977/DK/NIDDK NIH HHS/United States
- R37 DK030292/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases