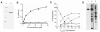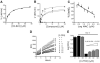PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release - PubMed (original) (raw)
. 2008 Nov 28;322(5906):1392-5.
doi: 10.1126/science.1164571.
Ujjini Manjunatha, Helena I M Boshoff, Young Hwan Ha, Pornwaratt Niyomrattanakit, Richard Ledwidge, Cynthia S Dowd, Ill Young Lee, Pilho Kim, Liang Zhang, Sunhee Kang, Thomas H Keller, Jan Jiricek, Clifton E Barry 3rd
Affiliations
- PMID: 19039139
- PMCID: PMC2723733
- DOI: 10.1126/science.1164571
PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release
Ramandeep Singh et al. Science. 2008.
Abstract
Bicyclic nitroimidazoles, including PA-824, are exciting candidates for the treatment of tuberculosis. These prodrugs require intracellular activation for their biological function. We found that Rv3547 is a deazaflavin-dependent nitroreductase (Ddn) that converts PA-824 into three primary metabolites; the major one is the corresponding des-nitroimidazole (des-nitro). When derivatives of PA-824 were used, the amount of des-nitro metabolite formed was highly correlated with anaerobic killing of Mycobacterium tuberculosis (Mtb). Des-nitro metabolite formation generated reactive nitrogen species, including nitric oxide (NO), which are the major effectors of the anaerobic activity of these compounds. Furthermore, NO scavengers protected the bacilli from the lethal effects of the drug. Thus, these compounds may act as intracellular NO donors and could augment a killing mechanism intrinsic to the innate immune system.
Figures
Fig. 1
Ddn-mediated activation of PA-824. (A) Ddn was expressed as a maltose-binding protein fusion in E. coli and purified on an amylose resin column. (B) Ddn-catalyzed oxidation of F420H2 using PA-824 as substrate (squares, the maximum number of substrate molecules an enzyme can turn over per unit of time, _k_cat)/_K_m = 0.145 min-1 μM-1,orthe enantiomer of PA-824 (inverted triangles _k_cat/_K_m = 0.016 min-1 μM-1). (C) Reaction was initiated by the addition of the indicated amount of F420H2, followed by incubation and analysis of aliquots by LC-MS. Squares, PA-824 (m/z 360); diamonds, des-nitro PA-824 (1, m/z 315); circles, metabolite 3 (m/z 291); inverted triangles, metabolite 2 (m/z 331); triangles, m/z 346, an unstable intermediate (C) quickly convertedto 3. (D) Thin-layer chromatography (TLC) analysis of the conversion of [14C]PA-824 (lane 1) by whole cells of Mtb (lane 2) or by purified Ddn using 25 μM F420H2 (lane 3). The numbered spots in (D) were shown to comigrate with the numbered metabolites from (C) by collecting HPLC peaks from (C) and analyzing by TLC.
Fig. 2
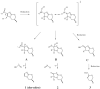
Mechanism of nitroimidazole reduction by Ddn. Initial hydride addition to C-3 of the bicyclic nitroimidazole results in formation of an intermediate that gives rise to all three observed stable products through the two resonance forms shown. Protonation of the C-2 position by solvent to give (A) followed by the elimination of nitrous acid to give 1. Hydrolysis gives (B) which leads to another loss of a reactive nitrogen species in an “enzymatic Nef reaction” to produce 2. Further reduction of the intermediate produces the unstable hydroxylamine (C) which decomposes to 3.
Fig.3
Formation of NO and correlation with anaerobic activity. (A) Monitoring of the in vitro reduction of PA-824 by Ddn using the Greiss reagent after incubation of purified Ddn with PA-824 and F420H2. **(B)**Kinetics of in vivo [NO] release in Bacille Calmette-Guérin (BCG)-sensitized cells with PA-824 (triangle), compound 10 (inverted triangle), compound 11 (square), isoniazid (diamond), and metronidazole (circle) by using DAF-FM diacetate. RFU, relative fluorescence units. (C) Correlation between anaerobic killing and the percentage of des-nitro metabolite formed by 8 bicyclic nitroimidazoles (Table 1). (D) In vivo [NO] release in BCG cells with 20 μM PA-824 in the presence of increasing CPTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide] concentration. (E) Protection of PA-824-mediated killing of nonreplicating hypoxic cells by C-PTIO. Error bars are SD of three independent replicates.
Comment in
- Microbiology. An antibiotic mimics immunity.
Nathan C. Nathan C. Science. 2008 Nov 28;322(5906):1337-8. doi: 10.1126/science.1167452. Science. 2008. PMID: 19039126 No abstract available.
Similar articles
- Microbiology. An antibiotic mimics immunity.
Nathan C. Nathan C. Science. 2008 Nov 28;322(5906):1337-8. doi: 10.1126/science.1167452. Science. 2008. PMID: 19039126 No abstract available. - Structure-activity relationships of antitubercular nitroimidazoles. 1. Structural features associated with aerobic and anaerobic activities of 4- and 5-nitroimidazoles.
Kim P, Zhang L, Manjunatha UH, Singh R, Patel S, Jiricek J, Keller TH, Boshoff HI, Barry CE 3rd, Dowd CS. Kim P, et al. J Med Chem. 2009 Mar 12;52(5):1317-28. doi: 10.1021/jm801246z. J Med Chem. 2009. PMID: 19209889 Free PMC article. - Substrate specificity of the deazaflavin-dependent nitroreductase from Mycobacterium tuberculosis responsible for the bioreductive activation of bicyclic nitroimidazoles.
Gurumurthy M, Mukherjee T, Dowd CS, Singh R, Niyomrattanakit P, Tay JA, Nayyar A, Lee YS, Cherian J, Boshoff HI, Dick T, Barry CE 3rd, Manjunatha UH. Gurumurthy M, et al. FEBS J. 2012 Jan;279(1):113-25. doi: 10.1111/j.1742-4658.2011.08404.x. Epub 2011 Nov 14. FEBS J. 2012. PMID: 22023140 Free PMC article. - The nitroimidazooxazines (PA-824 and analogs): structure-activity relationship and mechanistic studies.
Denny WA, Palmer BD. Denny WA, et al. Future Med Chem. 2010 Aug;2(8):1295-304. doi: 10.4155/fmc.10.207. Future Med Chem. 2010. PMID: 21426020 Review. - Molecule Property Analyses of Active Compounds for Mycobacterium tuberculosis.
Makarov V, Salina E, Reynolds RC, Kyaw Zin PP, Ekins S. Makarov V, et al. J Med Chem. 2020 Sep 10;63(17):8917-8955. doi: 10.1021/acs.jmedchem.9b02075. Epub 2020 Apr 20. J Med Chem. 2020. PMID: 32259446 Free PMC article. Review.
Cited by
- Efflux pumps and membrane permeability contribute to intrinsic antibiotic resistance in Mycobacterium abscessus.
McGowen K, Funck T, Wang X, Zinga S, Wolf ID, Akusobi CC, Denkinger CM, Rubin EJ, Sullivan MR. McGowen K, et al. bioRxiv [Preprint]. 2024 Aug 24:2024.08.23.609441. doi: 10.1101/2024.08.23.609441. bioRxiv. 2024. PMID: 39229117 Free PMC article. Preprint. - The pursuit of mechanism of action: uncovering drug complexity in TB drug discovery.
Yuan T, Werman JM, Sampson NS. Yuan T, et al. RSC Chem Biol. 2021 Apr 1;2(2):423-440. doi: 10.1039/d0cb00226g. Epub 2021 Jan 13. RSC Chem Biol. 2021. PMID: 33928253 Free PMC article. - Application of combined CRISPR screening for genetic and chemical-genetic interaction profiling in Mycobacterium tuberculosis.
Yan MY, Zheng D, Li SS, Ding XY, Wang CL, Guo XP, Zhan L, Jin Q, Yang J, Sun YC. Yan MY, et al. Sci Adv. 2022 Nov 25;8(47):eadd5907. doi: 10.1126/sciadv.add5907. Epub 2022 Nov 23. Sci Adv. 2022. PMID: 36417506 Free PMC article. - New insights into TB physiology suggest untapped therapeutic opportunities.
Baer CE, Rubin EJ, Sassetti CM. Baer CE, et al. Immunol Rev. 2015 Mar;264(1):327-43. doi: 10.1111/imr.12267. Immunol Rev. 2015. PMID: 25703570 Free PMC article. Review. - Activation of Bicyclic Nitro-drugs by a Novel Nitroreductase (NTR2) in Leishmania.
Wyllie S, Roberts AJ, Norval S, Patterson S, Foth BJ, Berriman M, Read KD, Fairlamb AH. Wyllie S, et al. PLoS Pathog. 2016 Nov 3;12(11):e1005971. doi: 10.1371/journal.ppat.1005971. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27812217 Free PMC article.
References
- Matteelli A, et al. Expert Rev. Anti Infect. Ther. 2007;5:857. - PubMed
- Barry CE, 3rd, Boshoff HI, Dowd CS. Curr. Pharm. Des. 2004;10:3239. - PubMed
- StopTB Working Group New Drugs. 2008. www.stoptb.org/wg/ new_drugs/assets/documents/2007GlobalPipeline.pdf.
- Stover CK, et al. Nature. 2000;405:962. - PubMed
- Boshoff HI, Barry CE., 3rd Nat. Rev. Microbiol. 2005;3:70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources