Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma - PubMed (original) (raw)
. 2009 Aug;13(8B):1977-1986.
doi: 10.1111/j.1582-4934.2008.00594.x.
Sven Perner 1, A John Iafrate 2, Beow Y Yeap 3, Barbara A Weir 4 5, Laura A Johnson 1, Bruce E Johnson 4, Matthew Meyerson 4 5, Mark A Rubin 1, William D Travis 6, Massimo Loda 1 5, Lucian R Chirieac 1
Affiliations
- PMID: 19040416
- PMCID: PMC2830395
- DOI: 10.1111/j.1582-4934.2008.00594.x
Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma
Justine A Barletta et al. J Cell Mol Med. 2009 Aug.
Abstract
The majority of lung adenocarcinomas express the lineage-specific thyroid transcription factor-1 (TTF-1). We recently reported that in a subset of lung adenocarcinomas the TTF-1 gene is amplified. Although the prognostic significance of TTF-1 expression has been previously investigated, the significance of TTF-1 amplification has not been established. We studied 89 consecutive patients with lung adenocarcinomas treated by surgery at Brigham and Women's Hospital between 1997 and 1999 and performed immunohistochemical analysis for TTF-1 expression and fluorescence in situ hybridization for TTF-1 amplification. We investigated associations between clinical-pathological characteristics, TTF-1 expression, TTF-1 amplification and overall survival. TTF-1 expression was categorized as high (48%), low (24%) or absent (28%). TTF-1 was amplified in 7% of cases. Patients with adenocarcinomas with low or high TTF-1 expression had a significantly better outcome than those with absent TTF-1 expression (median overall survival times of 72.4, 77.8 and 30.5 months, respectively, P = 0.002). In contrast, patients with adenocarcinomas with TTF-1 expression had a worse outcome if TTF-1 was amplified (median overall survival time 39.5 versus 87.5 months). In multivariate analysis, improved overall survival was independently predicted by TTF-1 expression in combination with no TTF-1 amplification (P < 0.001). In patients with lung adenocarcinoma, TTF-1 expression is a predictor of good outcome. Patients with no TTF-1 expression or TTF-1 expression and TTF-1 gene amplification tend to have a significantly worse prognosis than patients with TTF-1 expression and no TTF-1 gene amplification.
Figures
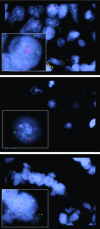
Figure 1
TTF1 amplification by fluorescence in situ hybridization (FISH). (A) FISH for NKX2–1 (TTF1, red) and a chromosome 14 reference probe (green) on a lung adenocarcinoma specimen with high‐level amplification of the NKX2–1 probe. Nuclei are stained with DAPI (blue). The box shows a single nucleus. (B) FISH on lung adenocarcinoma tissue without amplification. The boxed area shows a representative nucleus. (C) FISH for NKX2–1 (red) and a chromosome 14 reference probe (green) on a lung adenocarcinoma specimen with polysomy. Nuclei are stained with DAPI (blue). The box shows a single nucleus.
Figure 2
Diagram of representative tissue cores of the TMA illustrating the semi‐quantitative assessment of TTF‐1 expression in lung adenocarcinoma. TTF‐1 expression was semi‐quantitatively assigned to one of three categories: (A) No expression (absent staining = 0). (B) Low expression (weak staining intensity = 1), (C) High expression (strong staining intensity = 2).
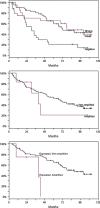
Figure 3
Kaplan–Meier estimates of overall survival among patients with lung adenocarcinoma according to TTF‐1 expression and TTF‐1 amplification. (A) The overall survival time was significantly longer for patients with adenocarcinomas with high or low TTF‐1 expression, than for patients with adenocarcinomas with absent TTF‐1 expression (_P_= 0.002). (B) There was no difference in overall survival between patients with adenocarcinomas with or without TTF‐1 gene amplification (_P_= 0.508). (C) There was a trend toward a worse overall survival for patients with adenocarcinomas with TTF‐1 expression and TTF‐1 gene amplification compared to patients with adenocarcinomas with TTF‐1 expression and without TTF‐1 amplification (median 39.5 versus 87.5 months, _P_= 0.113).
Similar articles
- The complexity of thyroid transcription factor 1 with both pro- and anti-oncogenic activities.
Mu D. Mu D. J Biol Chem. 2013 Aug 30;288(35):24992-25000. doi: 10.1074/jbc.R113.491647. Epub 2013 Jul 1. J Biol Chem. 2013. PMID: 23818522 Free PMC article. Review. - [Expression and clinical significance of the thyroid transcription factor 1 in Xuanwei lung adenocarcinoma].
Zu Y, Wang X, Liu X, Chen Y, Li C, Shang X, Xie Y, Zhao H, Wang J. Zu Y, et al. Zhongguo Fei Ai Za Zhi. 2013 Mar;16(3):131-7. doi: 10.3779/j.issn.1009-3419.2013.03.03. Zhongguo Fei Ai Za Zhi. 2013. PMID: 23514941 Free PMC article. Chinese. - Thyroid transcription factor-1 amplification and expressions in lung adenocarcinoma tissues and pleural effusions predict patient survival and prognosis.
Li X, Wan L, Shen H, Geng J, Nie J, Wang G, Jia N, Dai M, Bai X. Li X, et al. J Thorac Oncol. 2012 Jan;7(1):76-84. doi: 10.1097/JTO.0b013e318232b98a. J Thorac Oncol. 2012. PMID: 22011671 - Clinical significance of thyroid transcription factor-1 in advanced lung adenocarcinoma under epidermal growth factor receptor tyrosine kinase inhibitor treatment.
Chung KP, Huang YT, Chang YL, Yu CJ, Yang CH, Chang YC, Shih JY, Yang PC. Chung KP, et al. Chest. 2012 Feb;141(2):420-428. doi: 10.1378/chest.10-3149. Epub 2011 Jul 28. Chest. 2012. PMID: 21799026 - NKX2-1/TTF-1: an enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression.
Yamaguchi T, Hosono Y, Yanagisawa K, Takahashi T. Yamaguchi T, et al. Cancer Cell. 2013 Jun 10;23(6):718-23. doi: 10.1016/j.ccr.2013.04.002. Cancer Cell. 2013. PMID: 23763999 Review.
Cited by
- Nkx2-1 represses a latent gastric differentiation program in lung adenocarcinoma.
Snyder EL, Watanabe H, Magendantz M, Hoersch S, Chen TA, Wang DG, Crowley D, Whittaker CA, Meyerson M, Kimura S, Jacks T. Snyder EL, et al. Mol Cell. 2013 Apr 25;50(2):185-99. doi: 10.1016/j.molcel.2013.02.018. Epub 2013 Mar 21. Mol Cell. 2013. PMID: 23523371 Free PMC article. - Predicting Deep Learning Based Multi-Omics Parallel Integration Survival Subtypes in Lung Cancer Using Reverse Phase Protein Array Data.
Takahashi S, Asada K, Takasawa K, Shimoyama R, Sakai A, Bolatkan A, Shinkai N, Kobayashi K, Komatsu M, Kaneko S, Sese J, Hamamoto R. Takahashi S, et al. Biomolecules. 2020 Oct 19;10(10):1460. doi: 10.3390/biom10101460. Biomolecules. 2020. PMID: 33086649 Free PMC article. - The complexity of thyroid transcription factor 1 with both pro- and anti-oncogenic activities.
Mu D. Mu D. J Biol Chem. 2013 Aug 30;288(35):24992-25000. doi: 10.1074/jbc.R113.491647. Epub 2013 Jul 1. J Biol Chem. 2013. PMID: 23818522 Free PMC article. Review. - Pulmonary adenocarcinoma: implications of the recent advances in molecular biology, treatment and the IASLC/ATS/ERS classification.
Revannasiddaiah S, Thakur P, Bhardwaj B, Susheela SP, Madabhavi I. Revannasiddaiah S, et al. J Thorac Dis. 2014 Oct;6(Suppl 5):S502-25. doi: 10.3978/j.issn.2072-1439.2014.05.19. J Thorac Dis. 2014. PMID: 25349702 Free PMC article. Review. - Thyroid transcription factors in development, differentiation and disease.
Fernández LP, López-Márquez A, Santisteban P. Fernández LP, et al. Nat Rev Endocrinol. 2015 Jan;11(1):29-42. doi: 10.1038/nrendo.2014.186. Epub 2014 Oct 28. Nat Rev Endocrinol. 2015. PMID: 25350068 Review.
References
- Jemal A, Siegel R, Ward E, et al . Cancer statistics, 2008. CA Cancer J Clin. 2008; 58: 71–96. - PubMed
- Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest . 2007; 132: 29S–55S. - PubMed
- Ettinger DS, Bepler G, Bueno R, et al . Non‐small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006; 4: 548–82. - PubMed
- Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006; 11: 533–8. - PubMed
- Henschke CI, Yankelevitz DF, Libby DM, et al . Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006; 355: 1763–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 CA090578/CA/NCI NIH HHS/United States
- R01 CA092824/CA/NCI NIH HHS/United States
- CA074386/CA/NCI NIH HHS/United States
- CA90578/CA/NCI NIH HHS/United States
- P50 CA090578/CA/NCI NIH HHS/United States
- CA092824/CA/NCI NIH HHS/United States
- P20 CA090578-01A10003/CA/NCI NIH HHS/United States
- R01 CA074386/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical