Evolutionary origin and phylogenetic analysis of the novel oocyte-specific eukaryotic translation initiation factor 4E in Tetrapoda - PubMed (original) (raw)
Evolutionary origin and phylogenetic analysis of the novel oocyte-specific eukaryotic translation initiation factor 4E in Tetrapoda
Alexei V Evsikov et al. Dev Genes Evol. 2009 Feb.
Abstract
The transcriptionally active, growing oocyte accumulates mRNAs essential for early stages of development, the oocyte-to-embryo transition, in a stable, dormant form. Translational repression of mRNAs in eggs of various species is conferred by interactions, either direct or via intermediate proteins, of repressive factors bound to the 3'-untranslated regions with the proteins of the eukaryotic translation initiation factor 4E (eIF4E) family bound to the 5'-cap of the transcripts. Recently, a novel oocyte-specific eIF4E encoded by the Eif41b gene in mammals has been identified by our group. To further investigate this gene, the available cDNA libraries, as well as genome assemblies of nonmammalian vertebrates, were surveyed. This analysis revealed that the Eif4e1b gene arose in Tetrapoda as a result of the ancestral Eif4e locus duplication. Unlike other known proteins of three subfamilies comprising eIF4E family (eIF4E1, eIF4E2, and eIF4E3), cDNA library evidence suggests that Eif41b locus has an oocyte-restricted expression across all classes of Tetrapoda. To further understand the role of eIF4E1B during oocyte maturation, injections of antisense morpholino nucleotides in the X. tropicalis fully-grown stage VI oocytes were performed. The resulted ablation of eIF4E1B protein led to significant acceleration of oocyte maturation after progesterone induction; morpholino-injected oocytes formed the metaphase plate 30 min faster than the control groups. These results suggest that eIF4E1B protein acts as a repressor in translational regulation of maternal mRNAs activated during, and required for, oocyte maturation.
Figures
Figure 1. Conservation of genomic structure for Eif4e and Eif4e1b loci in Tetrapoda
Colinearity of genes underscores the common phylogenetic origins for Eif4e (a) and Eif4e1b (b) in tetrapods. (c) The Danio rerio eif4e1b locus is not conserved among Actinopterygii genomes. (d) While the colinearity of Sncb and Tspan17 orthologs persists across vertebrates, fish genomes lack the Eif4e1b locus. Orthologs shown in the same color; non-orthologous genes shown in black.
Figure 2. Actinopterigyii genomes contain multiple members of eIF4E1 subfamily
Danio rerio expresses at least four members of this family (Suppl. Table 1), three of which have orthologs in other sequenced fish genomes. Genomic arrangement of loci for putative orthologs of zebrafish eif4e1a (Eif4e1_1) (a), zgc:101581 (Eif4e1_2) (b) and zgc:110154 (Eif4e1_3) (c) genes. Orthologs shown in the same color; non-orthologous genes shown in black.
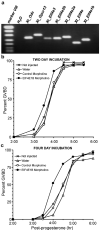
Figure 3. Gene expression of Eif4e1b and its function in oocytes of Xenopus tropicalis
(a) RT-PCR detection of eIF4E-encoding genes (Xt_Eif4e1b, Xt_Eif4e, Xt_Eif4e2a, Xt_Eif4e2b, Xt_Eif4e3), Xt-Gpcr13, Xt-Odc as positive controls of cDNA quality and H2O as a negative control. (b, c) Effects of blocking eIF4E1B translation on the resumption of meiosis and maturation in oocytes exposed to anti-Eif4e1b morpholino or control for two and four days.
Similar articles
- Distinct recruitment of human eIF4E isoforms to processing bodies and stress granules.
Frydryskova K, Masek T, Borcin K, Mrvova S, Venturi V, Pospisek M. Frydryskova K, et al. BMC Mol Biol. 2016 Aug 30;17(1):21. doi: 10.1186/s12867-016-0072-x. BMC Mol Biol. 2016. PMID: 27578149 Free PMC article. - CPEB interacts with an ovary-specific eIF4E and 4E-T in early Xenopus oocytes.
Minshall N, Reiter MH, Weil D, Standart N. Minshall N, et al. J Biol Chem. 2007 Dec 28;282(52):37389-401. doi: 10.1074/jbc.M704629200. Epub 2007 Oct 17. J Biol Chem. 2007. PMID: 17942399 - Selective Translation of Maternal mRNA by eIF4E1B Controls Oocyte to Embryo Transition.
Guo J, Zhao H, Zhang J, Lv X, Zhang S, Su R, Zheng W, Dai J, Meng F, Gong F, Lu G, Xue Y, Lin G. Guo J, et al. Adv Sci (Weinh). 2023 Apr;10(11):e2205500. doi: 10.1002/advs.202205500. Epub 2023 Feb 8. Adv Sci (Weinh). 2023. PMID: 36755190 Free PMC article. - eIF4E activity is regulated at multiple levels.
Raught B, Gingras AC. Raught B, et al. Int J Biochem Cell Biol. 1999 Jan;31(1):43-57. doi: 10.1016/s1357-2725(98)00131-9. Int J Biochem Cell Biol. 1999. PMID: 10216943 Review. - Structure and functions of the translation initiation factor eIF4E and its role in cancer development and treatment.
Piserà A, Campo A, Campo S. Piserà A, et al. J Genet Genomics. 2018 Jan 20;45(1):13-24. doi: 10.1016/j.jgg.2018.01.003. Epub 2018 Feb 1. J Genet Genomics. 2018. PMID: 29396141 Review.
Cited by
- The Eukaryotic Translation Initiation Factor 4E (eIF4E) as a Therapeutic Target for Cancer.
Karaki S, Andrieu C, Ziouziou H, Rocchi P. Karaki S, et al. Adv Protein Chem Struct Biol. 2015;101:1-26. doi: 10.1016/bs.apcsb.2015.09.001. Epub 2015 Oct 23. Adv Protein Chem Struct Biol. 2015. PMID: 26572974 Free PMC article. Review. - eIF4E: new family members, new binding partners, new roles.
Rhoads RE. Rhoads RE. J Biol Chem. 2009 Jun 19;284(25):16711-16715. doi: 10.1074/jbc.R900002200. Epub 2009 Feb 23. J Biol Chem. 2009. PMID: 19237539 Free PMC article. Review. - Diversity of Eukaryotic Translational Initiation Factor eIF4E in Protists.
Jagus R, Bachvaroff TR, Joshi B, Place AR. Jagus R, et al. Comp Funct Genomics. 2012;2012:134839. doi: 10.1155/2012/134839. Epub 2012 Jun 20. Comp Funct Genomics. 2012. PMID: 22778692 Free PMC article. - KHDC1B is a novel CPEB binding partner specifically expressed in mouse oocytes and early embryos.
Cai C, Tamai K, Molyneaux K. Cai C, et al. Mol Biol Cell. 2010 Sep 15;21(18):3137-48. doi: 10.1091/mbc.E10-03-0255. Epub 2010 Jul 28. Mol Biol Cell. 2010. PMID: 20668163 Free PMC article. - On the Diversification of the Translation Apparatus across Eukaryotes.
Hernández G, Proud CG, Preiss T, Parsyan A. Hernández G, et al. Comp Funct Genomics. 2012;2012:256848. doi: 10.1155/2012/256848. Epub 2012 May 14. Comp Funct Genomics. 2012. PMID: 22666084 Free PMC article.
References
- Bachvarova RF. A maternal tail of poly(A): the long and the short of it. Cell. 1992;69:895–897. - PubMed
- Carruthers S, Stemple DL. Genetic and genomic prospects for Xenopus tropicalis research. Semin Cell Dev Biol. 2006;17:146–153. - PubMed
- De Sousa PA, Masui Y. The effect of cytochalasin D on protein synthesis in Xenopus laevis oocytes. Mol Reprod Dev. 1990;26:248–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK 73267/DK/NIDDK NIH HHS/United States
- R03 DK073267/DK/NIDDK NIH HHS/United States
- R21 AA017244-01/AA/NIAAA NIH HHS/United States
- R21 AA017244/AA/NIAAA NIH HHS/United States
- R03 DK073267-02/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous