Dicer, Drosha, and outcomes in patients with ovarian cancer - PubMed (original) (raw)
Comparative Study
. 2008 Dec 18;359(25):2641-50.
doi: 10.1056/NEJMoa0803785.
Yvonne G Lin, Liz Y Han, Aparna A Kamat, Whitney A Spannuth, Rosemarie Schmandt, Diana Urbauer, Len A Pennacchio, Jan-Fang Cheng, Alpa M Nick, Michael T Deavers, Alexandra Mourad-Zeidan, Hua Wang, Peter Mueller, Marc E Lenburg, Joe W Gray, Samuel Mok, Michael J Birrer, Gabriel Lopez-Berestein, Robert L Coleman, Menashe Bar-Eli, Anil K Sood
Affiliations
- PMID: 19092150
- PMCID: PMC2710981
- DOI: 10.1056/NEJMoa0803785
Comparative Study
Dicer, Drosha, and outcomes in patients with ovarian cancer
William M Merritt et al. N Engl J Med. 2008.
Erratum in
- N Engl J Med. 2010 Nov 4;363(19):1877
Abstract
Background: We studied Dicer and Drosha, components of the RNA-interference machinery, in ovarian cancer.
Methods: We measured messenger RNA (mRNA) levels of Dicer and Drosha in specimens of invasive epithelial ovarian cancer from 111 patients, using a quantitative reverse-transcriptase-polymerase-chain-reaction assay, and compared the results with clinical outcomes. Validation was performed with the use of published microarray data from cohorts of patients with ovarian, breast, and lung cancer. Mutational analyses of genomic DNA from the Dicer and Drosha genes were performed in a subgroup of ovarian-cancer specimens. Dicer-dependent functional assays were performed by means of in vitro transfection with small interfering RNA (siRNA) and short hairpin RNA (shRNA).
Results: Levels of Dicer and Drosha mRNA correlated with the levels of expression of the corresponding protein and were decreased in 60% and 51% of ovarian-cancer specimens, respectively. Low Dicer expression was significantly associated with advanced tumor stage (P=0.007), and low Drosha expression with suboptimal surgical cytoreduction (P=0.02). Cancer specimens with both high Dicer expression and high Drosha expression were associated with increased median survival (>11 years, vs. 2.66 years for other subgroups; P<0.001). We found three independent predictors of reduced disease-specific survival in multivariate analyses: low Dicer expression (hazard ratio, 2.10; P=0.02), high-grade histologic features (hazard ratio, 2.46; P=0.03), and poor response to chemotherapy (hazard ratio, 3.95; P<0.001). Poor clinical outcomes among patients with low Dicer expression were validated in additional cohorts of patients. Rare missense mutations were found in the Dicer and Drosha genes, but their presence or absence did not correlate with the level of expression. Functional assays indicated that gene silencing with shRNA, but not siRNA, may be impaired in cells with low Dicer expression.
Conclusions: Our findings indicate that levels of Dicer and Drosha mRNA in ovarian-cancer cells have associations with outcomes in patients with ovarian cancer.
2008 Massachusetts Medical Society
Conflict of interest statement
Dr. Gray reports receiving consulting fees from Agendia and Sirna. No other potential conflict of interest relevant to this article was reported.
Figures
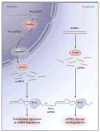
Figure 1. The RNA-Interference Cascade in Humans
Long precursor microRNA (miRNA) segments, called pri-miRNA, are first cleaved in the nucleus by Drosha, an RNase III endonuclease, into segments of approximately 70 nucleotides each (called pre-miRNA). Transportation into the cytoplasm by means of exportin 5 leads to cleavage by Dicer, another RNase III endonuclease, which produces mature miRNA segments. Host degradation of messenger RNA (mRNA) and translational repression occurs after miRNA binds to the RNA-induced silencing complex (RISC). Cytoplasmic long double-stranded RNA (dsRNA) is cleaved by Dicer into small interfering RNA (siRNA), which is incorporated into RISC, resulting in the cleavage and degradation of specific target mRNA.
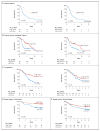
Figure 2. Kaplan–Meier Survival Curves for Patients According to Tumor Expression of Dicer and Drosha
For patients with invasive epithelial ovarian cancer, curves are shown for Dicer and Drosha expression (Panel A), with curves for Dicer and Drosha combined shown for comparison in Fig. 3 in the Supplementary Appendix. Curves from validation analyses are also shown for the expression of Dicer and Drosha in independent cohorts of patients with ovarian cancer (Panel B) and lung cancer (Panel C), as well as Dicer expression in two cohorts of patients with breast cancer (Panels D and E).
Figure 3. Transfection of Small Interfering RNA (siRNA) and Short Hairpin RNA (shRNA) Targeting Galectin-3 in Ovarian-Cancer Cell Lines with Low Dicer Expression and Those with High Dicer Expression
Western blot densitometry analysis was performed for the cell lines transfected with siRNA or shRNA or control (nontargeting) sequences. Actin was used for purposes of normalization.
Comment in
- MicroRNA in cancer prognosis.
Slack FJ, Weidhaas JB. Slack FJ, et al. N Engl J Med. 2008 Dec 18;359(25):2720-2. doi: 10.1056/NEJMe0808667. N Engl J Med. 2008. PMID: 19092157 Free PMC article. No abstract available. - Dicer and outcomes in patients with acute myeloid leukemia (AML).
Martin MG, Payton JE, Link DC. Martin MG, et al. Leuk Res. 2009 Aug;33(8):e127. doi: 10.1016/j.leukres.2009.02.003. Epub 2009 Mar 10. Leuk Res. 2009. PMID: 19278725 No abstract available. - Dicer and Drosha in ovarian cancer.
Köbel M, Gilks CB, Huntsman DG. Köbel M, et al. N Engl J Med. 2009 Mar 12;360(11):1150-1; author reply 1151. doi: 10.1056/NEJMc090085. N Engl J Med. 2009. PMID: 19279349 No abstract available.
Similar articles
- Dicer and outcomes in patients with acute myeloid leukemia (AML).
Martin MG, Payton JE, Link DC. Martin MG, et al. Leuk Res. 2009 Aug;33(8):e127. doi: 10.1016/j.leukres.2009.02.003. Epub 2009 Mar 10. Leuk Res. 2009. PMID: 19278725 No abstract available. - Argonaute, Dicer, and Drosha are up-regulated along tumor progression in serous ovarian carcinoma.
Vaksman O, Hetland TE, Trope' CG, Reich R, Davidson B. Vaksman O, et al. Hum Pathol. 2012 Nov;43(11):2062-9. doi: 10.1016/j.humpath.2012.02.016. Epub 2012 May 29. Hum Pathol. 2012. PMID: 22647351 - Dicer and Drosha expression and response to Bevacizumab-based therapy in advanced colorectal cancer patients.
Vincenzi B, Zoccoli A, Schiavon G, Iuliani M, Pantano F, Dell'aquila E, Ratta R, Muda AO, Perrone G, Brunelli C, Correale P, Riva E, Russo A, Loupakis F, Falcone A, Santini D, Tonini G. Vincenzi B, et al. Eur J Cancer. 2013 Apr;49(6):1501-8. doi: 10.1016/j.ejca.2012.11.014. Epub 2012 Dec 22. Eur J Cancer. 2013. PMID: 23266047 - Dicer cuts the kidney.
Ho JJ, Marsden PA. Ho JJ, et al. J Am Soc Nephrol. 2008 Nov;19(11):2043-6. doi: 10.1681/ASN.2008090986. Epub 2008 Oct 15. J Am Soc Nephrol. 2008. PMID: 18923053 Review. No abstract available. - The dicey role of Dicer: implications for RNAi therapy.
Merritt WM, Bar-Eli M, Sood AK. Merritt WM, et al. Cancer Res. 2010 Apr 1;70(7):2571-4. doi: 10.1158/0008-5472.CAN-09-2536. Epub 2010 Feb 23. Cancer Res. 2010. PMID: 20179193 Free PMC article. Review.
Cited by
- An mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose- and amino acid-deprivation.
Ye P, Liu Y, Chen C, Tang F, Wu Q, Wang X, Liu CG, Liu X, Liu R, Liu Y, Zheng P. Ye P, et al. Mol Cell. 2015 Feb 19;57(4):708-720. doi: 10.1016/j.molcel.2014.12.034. Epub 2015 Jan 29. Mol Cell. 2015. PMID: 25639470 Free PMC article. - A miR-192-EGR1-HOXB9 regulatory network controls the angiogenic switch in cancer.
Wu SY, Rupaimoole R, Shen F, Pradeep S, Pecot CV, Ivan C, Nagaraja AS, Gharpure KM, Pham E, Hatakeyama H, McGuire MH, Haemmerle M, Vidal-Anaya V, Olsen C, Rodriguez-Aguayo C, Filant J, Ehsanipour EA, Herbrich SM, Maiti SN, Huang L, Kim JH, Zhang X, Han HD, Armaiz-Pena GN, Seviour EG, Tucker S, Zhang M, Yang D, Cooper LJ, Ali-Fehmi R, Bar-Eli M, Lee JS, Ram PT, Baggerly KA, Lopez-Berestein G, Hung MC, Sood AK. Wu SY, et al. Nat Commun. 2016 Apr 4;7:11169. doi: 10.1038/ncomms11169. Nat Commun. 2016. PMID: 27041221 Free PMC article. - Micro-RNAs as diagnostic or prognostic markers in human epithelial malignancies.
Hui A, How C, Ito E, Liu FF. Hui A, et al. BMC Cancer. 2011 Nov 30;11:500. doi: 10.1186/1471-2407-11-500. BMC Cancer. 2011. PMID: 22128797 Free PMC article. Review. - The role of dicer in DNA damage repair.
Tang KF, Ren H. Tang KF, et al. Int J Mol Sci. 2012 Dec 7;13(12):16769-78. doi: 10.3390/ijms131216769. Int J Mol Sci. 2012. PMID: 23222681 Free PMC article. Review. - Sjogren syndrome antigen B (SSB)/La promotes global microRNA expression by binding microRNA precursors through stem-loop recognition.
Liang C, Xiong K, Szulwach KE, Zhang Y, Wang Z, Peng J, Fu M, Jin P, Suzuki HI, Liu Q. Liang C, et al. J Biol Chem. 2013 Jan 4;288(1):723-36. doi: 10.1074/jbc.M112.401323. Epub 2012 Nov 5. J Biol Chem. 2013. PMID: 23129761 Free PMC article.
References
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. - PubMed
- Landen CN, Jr, Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–8. - PubMed
- Hannon GJ. RNA interference. Nature. 2002;418:244–51. - PubMed
- Lee Y, Ahn C, Han J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA109298/CA/NCI NIH HHS/United States
- R01 CA110793/CA/NCI NIH HHS/United States
- CA110793/CA/NCI NIH HHS/United States
- U54 CA112970/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- P50 CA083639-090008/CA/NCI NIH HHS/United States
- T32 CA101642/CA/NCI NIH HHS/United States
- CA109298/CA/NCI NIH HHS/United States
- U54 CA112970-05/CA/NCI NIH HHS/United States
- P50 CA58207/CA/NCI NIH HHS/United States
- P50 CA058207/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical