Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters - PubMed (original) (raw)
Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters
Sergia Bortolanza et al. Mol Ther. 2009 Apr.
Abstract
Pancreatic cancer is an aggressive malignancy resistant to most conventional and experimental therapies, including conditionally replicative adenoviruses (CRAds). The incorporation of immunostimulatory genes such as interleukin-12 (IL-12) in these viruses may overcome some of their limitations, but evaluation of such vectors requires suitable preclinical models. We describe a CRAd in which replication is dependent on hypoxia-inducible factor (HIF) activity and alterations of the pRB pathway in cancer cells. Transgenes (luciferase or IL-12) were incorporated into E3 region of the virus using a selective 6.7K/gp19K deletion. A novel permissive model of pancreatic cancer developed in immunocompetent Syrian hamsters was used for in vivo analysis. We show that, in contrast with nonreplicating adenoviruses (NR-Ad), active viral production and enhanced transgene expression took place in vivo. A single intratumor inoculation of the CRAd expressing IL-12 (Ad-DHscIL12) achieved a potent antitumor effect, whereas higher doses of replication-competent adenoviruses carrying luciferase did not. Compared to a standard NR-Ad expressing IL-12, Ad-DHscIL12 was less toxic in hamsters, with more selective tumor expression and shorter systemic exposure to the cytokine. We conclude that the expression of IL-12 in the context of a hypoxia-inducible oncolytic adenovirus is effective against pancreatic cancer in a relevant animal model.
Figures
**Figure 1
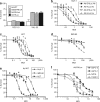
Influence of hypoxia on the activity of hypoxia-inducible factor (HIF)-dependent conditionally replicative adenoviruses. (a) Schematic representation of the genomes of replication-competent adenoviruses used in this study. (b) Western blot of fiber in human cancer (HuH-7 and A549) and normal (IMR-90) cells infected with Ad-DHLuc (DH), Ad-HLuc (H), and Ad-WT (WT). Infection was carried out for 2 hours at MOI 10 and 100 for cancer and normal cells, respectively. Cells were then washed and incubated with fresh culture medium in normoxic (Nx) or hypoxic condition (1% O2, Hx). Cell extracts were obtained 2 days later. (c) Western blot of HIF-1α in human HuH-7, A549, IMR-90, and hamster H2T cells maintained under normoxic or hypoxic conditions during 12 hours. Glyceraldehyde-3-phosphate dehydrogenase is shown to verify homogeneous loading of samples.
**Figure 2
Cytolytic effect of viruses. (a) Cells with normal or constitutively activated HIF pathways (VHL53 and RCC-10, respectively) were infected with Ad-DHLuc, Ad-HLuc, Ad-WT, or Ad9xHRE1A viruses and maintained for 7 days under normoxic conditions until viability was determined. The percentage of surviving cells versus noninfected controls is represented. (b–d) Influence of the E1A CR2 deletion. (b) HuH-7, (c) H2T, and (d) IMR90 cells were infected with Ad-DHLuc or Ad-HLuc at the indicated MOIs and cultured under normoxia (Nx, black symbols) or hypoxia (Hx, white symbols) for 5 (HuH-7, H2T) or 9 days (IMR-90). (e) Cytolytic effect of the Ad-WT on HuH-7, H2T, and IMR-90 cells under normoxic conditions. (f) Cytolytic effect of Ad-DHLuc on human pancreatic cancer cell lines BxPC-3, AsPC-1, NP18, and PANC-1 under normoxic conditions. Viability was determined 5 days after infection in e and f panels. The percentage of surviving cells versus noninfected controls is represented as a sigmoidal dose–response curve. Each curve is a representative experiment from at least four performed in triplicate.
**Figure 3
Stimulation of viral replication by hypoxia in normal and tumor cells. Cells were infected for 2 hours with Ad-DHLuc, Ad-Hluc, and Ad-WT at MOI 10 (HuH-7) or 100 (IMR-90). Then virus was removed and cells were washed and maintained in normoxia (Nx) or hypoxia (Hx) for 48 hours. The amount of infective virus extracted from cell lysates is represented as iu/cell. *P < 0.05, ***P < 0.0001.
**Figure 4
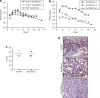
Transgene expression and replication of Ad-DHLuc in vivo. (a) Tumor xenografts were induced in the flank of nu/nu mice by subcutaneous inoculation of HuH-7 or H2T cells. When tumors reached at least 150 mm3 volume, a single dose of 2 × 108 iu Ad-DHLuc was injected intratumorally. The same dose of Ad-CMVLuc was injected in HuH-7 xenografts for comparison. Luciferase activity was analyzed over time and quantified (in photons/s) using an in vivo bioluminescence detection system. (b) Liver metastases of pancreatic cancer were established in Syrian hamsters by intrahepatic injection of H2T cells. One month later, 2 × 109 iu of Ad-DHLuc (squares), or Ad-CMVLuc (triangles, dotted line) were injected intratumorally (n = 5). In a separate group, healthy hamsters were inoculated intrahepatically with Ad-DHLuc (circles). Luciferase activity was measured daily. (c,d) Pancreatic tumors were obtained as indicated for panel b. Ad-DHLuc or Ad-WT were inoculated intratumorally at 2 × 109 iu/hamster. Four days later, animals were sacrificed and each tumor was divided in two. One half was lyzed for determination of viable viral particles and is represented as iu/mg tumor for each animal (c). The other portion was processed for detection of adenoviral capsid proteins in frozen sections as indicated (d). Positive cells display brown staining. A section from a tumor inoculated with the same dose of Ad-CMVLuc is included as a negative control for replication. Representative pictures at ×400 magnification are shown.
**Figure 5
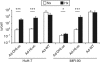
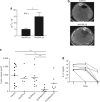
Biological activity and antitumor effect of Ad-DHscIL12 in liver metastases of pancreatic cancer in hamsters. Liver metastases of pancreatic cancer were established in Syrian hamsters by intrahepatic injection of H2T cells. Five weeks later, tumors were inoculated with a single dose of Ad-DHscIL12 or Ad-DHLuc at 2 × 107 iu; Ad-WTLuc at 2 × 109 iu, Ad-CMVscIL12 at 107 iu, or saline solution. (a) Some animals (n = 4) treated with Ad-WTLuc or Ad-DHscIL12 were sacrificed 3 days after vector administration, and expression of hamster interferon (IFN)-γ was analyzed by quantitative reverse transcriptase-PCR in tumor samples. (b) One month after virus inoculation, abdominal micro-CT was performed in hamsters treated with Ad-DHscIL12 or saline as indicated. Horizontal sections of representative animals are shown. Arrows indicate the margins of the tumor. (c) All animals were then sacrificed and tumor volume was directly measured at necropsy. (d) Serum was collected at days 1, 3, and 5 after virus injection, and murine interleukin (IL)-12 was determined by ELISA. White and black triangles represent animals that experienced complete or partial antitumor effect at the end of the experiment, respectively. *P < 0.05, ** P < 0.01.
**Figure 6
Specificity of interleukin (IL)-12 expression and toxicity of Ad-DHscIL12 and Ad-CMV-scIL12 viruses. Liver metastases of pancreatic cancer were established in Syrian hamsters by intrahepatic injection of H2T cells. Five weeks later, tumors were inoculated with a single dose of Ad-DHscIL12 at 2 × 107 iu (squares), or Ad-CMV-scIL12 at 2 × 107 iu (triangles) or 107 iu (circles). (a) One set of animals corresponding to the high-dose groups (n = 5) were sacrificed 3 days after virus inoculation for organ collection. The concentration of IL-12 was measured by ELISA in homogenates from tumors, livers, and lungs, as indicated. Values are expressed as ng IL-12/g tissue. (b) The rest of the animals (n = 8 in each group), were monitored for survival. *P = 0.019, **P = 0.002, log-rank test. (c) Histological analysis of lung, colon, and liver from a moribund hamster 6 days after treatment with 2 × 107 iu of Ad-CMV-scIL12, compared with a control animal inoculated with saline solution. Inflammatory infiltration and edema found in the lungs of the vector-treated hamster are marked by arrows and asterisk, respectively (Hematoxylin-Eosin staining of paraffin-embedded sections, ×200 magnification). (d) Concentration of IL-12 in serum determined at days 1, 3, and 5.
**Figure 7
Ad-DHscIL12-treated hamsters develop H2T-specific immune responses. (a) Hamsters bearing H2T tumors were treated with Ad-DHscIL12 (continuous line) or Ad-CMV-scIL12 (dotted line). Twenty days later, peripheral blood mononuclear cell from these animals were labeled with CFSE and incubated with irradiated H2T cells for 3 days. Histogram displaying fluorescence intensity of leukocytes from one representative animal in each group is represented. (b) Average proliferation index of each group (n = 3). *P < 0.05.
Similar articles
- Deletion of the E3-6.7K/gp19K region reduces the persistence of wild-type adenovirus in a permissive tumor model in Syrian hamsters.
Bortolanza S, Bunuales M, Alzuguren P, Lamas O, Aldabe R, Prieto J, Hernandez-Alcoceba R. Bortolanza S, et al. Cancer Gene Ther. 2009 Sep;16(9):703-12. doi: 10.1038/cgt.2009.12. Epub 2009 Feb 20. Cancer Gene Ther. 2009. PMID: 19229289 - Evaluation of monocytes as carriers for armed oncolytic adenoviruses in murine and Syrian hamster models of cancer.
Bunuales M, Garcia-Aragoncillo E, Casado R, Quetglas JI, Hervas-Stubbs S, Bortolanza S, Benavides-Vallve C, Ortiz-de-Solorzano C, Prieto J, Hernandez-Alcoceba R. Bunuales M, et al. Hum Gene Ther. 2012 Dec;23(12):1258-68. doi: 10.1089/hum.2012.043. Epub 2012 Oct 26. Hum Gene Ther. 2012. PMID: 22985305 Free PMC article. - Enhanced therapeutic effect using sequential administration of antigenically distinct oncolytic viruses expressing oncostatin M in a Syrian hamster orthotopic pancreatic cancer model.
Nistal-Villan E, Bunuales M, Poutou J, Gonzalez-Aparicio M, Bravo-Perez C, Quetglas JI, Carte B, Gonzalez-Aseguinolaza G, Prieto J, Larrea E, Hernandez-Alcoceba R. Nistal-Villan E, et al. Mol Cancer. 2015 Dec 16;14:210. doi: 10.1186/s12943-015-0479-x. Mol Cancer. 2015. PMID: 26671477 Free PMC article. - Armed replicating adenoviruses for cancer virotherapy.
Cody JJ, Douglas JT. Cody JJ, et al. Cancer Gene Ther. 2009 Jun;16(6):473-88. doi: 10.1038/cgt.2009.3. Epub 2009 Feb 6. Cancer Gene Ther. 2009. PMID: 19197323 Free PMC article. Review. - Chapter three--Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds.
Wold WS, Toth K. Wold WS, et al. Adv Cancer Res. 2012;115:69-92. doi: 10.1016/B978-0-12-398342-8.00003-3. Adv Cancer Res. 2012. PMID: 23021242 Review.
Cited by
- Interleukin-12 Delivery Strategies and Advances in Tumor Immunotherapy.
Dong C, Tan D, Sun H, Li Z, Zhang L, Zheng Y, Liu S, Zhang Y, He Q. Dong C, et al. Curr Issues Mol Biol. 2024 Oct 16;46(10):11548-11579. doi: 10.3390/cimb46100686. Curr Issues Mol Biol. 2024. PMID: 39451566 Free PMC article. Review. - Biomimetic Nucleic Acid Drug Delivery Systems for Relieving Tumor Immunosuppressive Microenvironment.
Yan W, Cao Y, Yin Q, Li Y. Yan W, et al. Pharmaceutics. 2024 Aug 1;16(8):1028. doi: 10.3390/pharmaceutics16081028. Pharmaceutics. 2024. PMID: 39204373 Free PMC article. Review. - Clinical Application of Adenovirus (AdV): A Comprehensive Review.
Salauddin M, Saha S, Hossain MG, Okuda K, Shimada M. Salauddin M, et al. Viruses. 2024 Jul 8;16(7):1094. doi: 10.3390/v16071094. Viruses. 2024. PMID: 39066256 Free PMC article. Review. - A viral attack on brain tumors: the potential of oncolytic virus therapy.
Mokhtarpour K, Akbarzadehmoallemkolaei M, Rezaei N. Mokhtarpour K, et al. J Neurovirol. 2024 Jun;30(3):229-250. doi: 10.1007/s13365-024-01209-8. Epub 2024 May 28. J Neurovirol. 2024. PMID: 38806994 Review. - Zika Virus: A Neurotropic Warrior against High-Grade Gliomas-Unveiling Its Potential for Oncolytic Virotherapy.
Calderón-Peláez MA, Maradei Anaya SJ, Bedoya-Rodríguez IJ, González-Ipuz KG, Vera-Palacios D, Buitrago IV, Castellanos JE, Velandia-Romero ML. Calderón-Peláez MA, et al. Viruses. 2024 Apr 3;16(4):561. doi: 10.3390/v16040561. Viruses. 2024. PMID: 38675903 Free PMC article. Review.
References
- Aghi M., and , Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24:7802–7816. - PubMed
- Le QT, Denko NC., and , Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. - PubMed
- Cuevas Y, Hernandez-Alcoceba R, Aragones J, Naranjo-Suarez S, Castellanos MC, Esteban MA, et al. Specific oncolytic effect of a new hypoxia-inducible factor-dependent replicative adenovirus on von Hippel-Lindau-defective renal cell carcinomas. Cancer Res. 2003;63:6877–6884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous