The role of a murine transplantation model of atherosclerosis regression in drug discovery - PubMed (original) (raw)
Review
The role of a murine transplantation model of atherosclerosis regression in drug discovery
Jonathan E Feig et al. Curr Opin Investig Drugs. 2009 Mar.
Abstract
Atherosclerosis is the leading cause of death worldwide. To date, the use of statins to lower LDL levels has been the major intervention used to delay or halt disease progression. These drugs have an incomplete impact on plaque burden and risk, however, as evidenced by the substantial rates of myocardial infarctions that occur in large-scale clinical trials of statins. Thus, it is hoped that by understanding the factors that lead to plaque regression, better approaches to treating atherosclerosis may be developed. A transplantation-based mouse model of atherosclerosis regression has been developed by allowing plaques to form in a model of human atherosclerosis, the apoE-deficient mouse, and then placing these plaques into recipient mice with a normolipidemic plasma environment. Under these conditions, the depletion of foam cells occurs. Interestingly, the disappearance of foam cells was primarily due to migration in a CCR7-dependent manner to regional and systemic lymph nodes after 3 days in the normolipidemic (regression) environment. Further studies using this transplant model demonstrated that liver X receptor and HDL are other factors likely to be involved in plaque regression. In conclusion, through the use of this transplant model, the process of uncovering the pathways regulating atherosclerosis regression has begun, which will ultimately lead to the identification of new therapeutic targets.
Figures
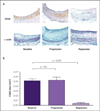
Figure 1. Regression of advanced atherosclerotic plaques in the mouse transplantation model
Mice null for apolipoprotein E were fed a Western diet for 40 weeks to promote advanced atherosclerosis. Aortic arches from these mice were either harvested and analyzed by (A) histochemical and (B) histomorphometric methods, or were transplanted into apolipoprotein E-null ('progression') or wild-type ('regression') recipient mice. After 9 weeks, the same analyses were conducted on the aortic arches from transplantation recipients. The histochemical results for the foam cell marker CD68 (brown) and the vascular smooth cell marker α-actin (purple) are illustrated. The images demonstrate the immunostaining of representative aortic plaques in cross section. Note the low levels of foam cells and the appearance of a fibrous cap in the regression group. In contrast to the results in the regression mice, the progression group demonstrated a persistence of foam cells and no development of a fibrous cap. As shown in panel B, the visual histochemical staining suggesting a large depletion of foam cells was confirmed by quantitative analysis of the plaque area stained for CD68. NS not significant (Adapted with permission from Lippincott Williams & Wilkins and Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JGS, Mani V, Fallon JT, Chereshnev I, Fisher EA: Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol (2004) 24(9):1714–1719. © 2004 Lippincott Williams & Wilkins)
Similar articles
- Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice.
Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Trogan E, et al. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3781-6. doi: 10.1073/pnas.0511043103. Epub 2006 Mar 1. Proc Natl Acad Sci U S A. 2006. PMID: 16537455 Free PMC article. - HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells.
Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. Feig JE, et al. Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):7166-71. doi: 10.1073/pnas.1016086108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482781 Free PMC article. - Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages.
Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Feig JE, et al. PLoS One. 2011;6(12):e28534. doi: 10.1371/journal.pone.0028534. Epub 2011 Dec 6. PLoS One. 2011. PMID: 22163030 Free PMC article. - Regression of atherosclerosis: insights from animal and clinical studies.
Feig JE. Feig JE. Ann Glob Health. 2014 Jan-Feb;80(1):13-23. doi: 10.1016/j.aogh.2013.12.001. Epub 2013 Dec 25. Ann Glob Health. 2014. PMID: 24751561 Free PMC article. Review. - The role of an experimental model of atherosclerosis: apoE-knockout mice in developing new drugs against atherogenesis.
Jawien J. Jawien J. Curr Pharm Biotechnol. 2012 Oct;13(13):2435-9. Curr Pharm Biotechnol. 2012. PMID: 22280417 Review.
Cited by
- Visualization of Monocytic Cells in Regressing Atherosclerotic Plaques by Intravital 2-Photon and Positron Emission Tomography-Based Imaging-Brief Report.
Li W, Luehmann HP, Hsiao HM, Tanaka S, Higashikubo R, Gauthier JM, Sultan D, Lavine KJ, Brody SL, Gelman AE, Gropler RJ, Liu Y, Kreisel D. Li W, et al. Arterioscler Thromb Vasc Biol. 2018 May;38(5):1030-1036. doi: 10.1161/ATVBAHA.117.310517. Epub 2018 Mar 22. Arterioscler Thromb Vasc Biol. 2018. PMID: 29567678 Free PMC article. - Acute perioperative-stress-induced increase of atherosclerotic plaque volume and vulnerability to rupture in apolipoprotein-E-deficient mice is amenable to statin treatment and IL-6 inhibition.
Janssen H, Wagner CS, Demmer P, Callies S, Sölter G, Loghmani-khouzani H, Hu N, Schuett H, Tietge UJ, Warnecke G, Larmann J, Theilmeier G. Janssen H, et al. Dis Model Mech. 2015 Sep;8(9):1071-80. doi: 10.1242/dmm.018713. Epub 2015 Jun 18. Dis Model Mech. 2015. PMID: 26092124 Free PMC article. - Insights From Pre-Clinical and Clinical Studies on the Role of Innate Inflammation in Atherosclerosis Regression.
Rahman K, Fisher EA. Rahman K, et al. Front Cardiovasc Med. 2018 May 11;5:32. doi: 10.3389/fcvm.2018.00032. eCollection 2018. Front Cardiovasc Med. 2018. PMID: 29868610 Free PMC article. - Specialized Pro-resolving Lipid Mediators: Modulation of Diabetes-Associated Cardio-, Reno-, and Retino-Vascular Complications.
de Gaetano M, McEvoy C, Andrews D, Cacace A, Hunter J, Brennan E, Godson C. de Gaetano M, et al. Front Pharmacol. 2018 Dec 19;9:1488. doi: 10.3389/fphar.2018.01488. eCollection 2018. Front Pharmacol. 2018. PMID: 30618774 Free PMC article. Review. - Inflammation and atherosclerosis: direct versus indirect mechanisms.
Rosenfeld ME. Rosenfeld ME. Curr Opin Pharmacol. 2013 Apr;13(2):154-60. doi: 10.1016/j.coph.2013.01.003. Epub 2013 Jan 26. Curr Opin Pharmacol. 2013. PMID: 23357128 Free PMC article. Review.
References
- Blankenhorn DH, Hodis HN. George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler Thromb. 1994;14(2):177–192. - PubMed
- Williams KJ, Feig JE, Fisher EA. Cellular and molecular mechanisms for rapid regression of atherosclerosis: From bench top to potentially achievable clinical goal. Curr Opin Lipidol. 2007;18(4):443–450. - PubMed
- Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: Insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5(2):91–102. - PubMed
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15(5):551–561. •• A provocative synthesis of the order of events in the initiation and subsequent progression of an atherosclerotic plaque.
- Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104(4):503–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL 084312/HL/NHLBI NIH HHS/United States
- F30 AG029748/AG/NIA NIH HHS/United States
- DK 087945/DK/NIDDK NIH HHS/United States
- K08 DK087945/DK/NIDDK NIH HHS/United States
- AG 029748/AG/NIA NIH HHS/United States
- R01 HL084312/HL/NHLBI NIH HHS/United States
- HL 61814/HL/NHLBI NIH HHS/United States
- T32 GM007308/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous