Genetic control of wayward pluripotent stem cells and their progeny after transplantation - PubMed (original) (raw)
Genetic control of wayward pluripotent stem cells and their progeny after transplantation
Maija Kiuru et al. Cell Stem Cell. 2009.
Abstract
The proliferative capacity of pluripotent stem cells and their progeny brings a unique aspect to therapeutics, in that once a transplant is initiated the therapist no longer has control of the therapy. In the context of the recent FDA approval of a human ESC trial and report of a neuronal-stem-cell-derived tumor in a human trial, strategies need to be developed to control wayward pluripotent stem cells. Here, we focus on one approach: direct genetic modification of the cells prior to transplantation with genes that can prevent the adverse events and/or eliminate the transplanted cells and their progeny.
Figures
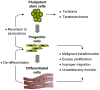
Figure 1. The Pluripotent Stem Cell Is the Factory to Generate the Progenitor and Differentiated Cells to Be Transplanted for Therapeutic Purposes
The risks of the various cell populations are indicated. The example is of differentiated cardiac muscle, but the paradigm holds for any differentiated cell type.
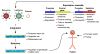
Figure 2. Genetic Engineering Strategies to Facilitate Control of Potential Wayward Pluripotent Stem Cells and Their Progeny
The vectors (retrovirus, lentivirus, or plasmid) mediate integration of the expression cassette into the pluripotent stem cell or its progeny depending on the application. The expression cassette contains two elements. One promoter (constitutive, pluripotent, or regulated depending on the need) regulates the “control” gene (pluripotent antisense, enzyme prodrug, toxin or apoptotic, or plasma membrane tag). The second promoter regulates a gene used for selection (antibiotic based or a plasma membrane tag). The stem cell population that has been genetically modified is then selected and transferred to the patient as dictated by the therapeutic needs. If there is a need to control a wayward stem cell population, the promoter is activated and/or a prodrug administered, eliminating the wayward cells.
Similar articles
- Safeguarding clinical translation of pluripotent stem cells with suicide genes.
Li W, Xiang AP. Li W, et al. Organogenesis. 2013 Jan-Mar;9(1):34-9. doi: 10.4161/org.24317. Epub 2013 Jan 1. Organogenesis. 2013. PMID: 23511011 Free PMC article. Review. - Selective ablation of human embryonic stem cells expressing a "suicide" gene.
Schuldiner M, Itskovitz-Eldor J, Benvenisty N. Schuldiner M, et al. Stem Cells. 2003;21(3):257-65. doi: 10.1634/stemcells.21-3-257. Stem Cells. 2003. PMID: 12743320 - Protecting against wayward human induced pluripotent stem cells with a suicide gene.
Cheng F, Ke Q, Chen F, Cai B, Gao Y, Ye C, Wang D, Zhang L, Lahn BT, Li W, Xiang AP. Cheng F, et al. Biomaterials. 2012 Apr;33(11):3195-204. doi: 10.1016/j.biomaterials.2012.01.023. Epub 2012 Jan 24. Biomaterials. 2012. PMID: 22269649 - Human stem cells harboring a suicide gene improve the safety and standardisation of neural transplants in Parkinsonian rats.
de Luzy IR, Law KCL, Moriarty N, Hunt CPJ, Durnall JC, Thompson LH, Nagy A, Parish CL. de Luzy IR, et al. Nat Commun. 2021 May 27;12(1):3275. doi: 10.1038/s41467-021-23125-9. Nat Commun. 2021. PMID: 34045451 Free PMC article. - Embryonic stem cell microRNAs: defining factors in induced pluripotent (iPS) and cancer (CSC) stem cells?
Gunaratne PH. Gunaratne PH. Curr Stem Cell Res Ther. 2009 Sep;4(3):168-77. doi: 10.2174/157488809789057400. Curr Stem Cell Res Ther. 2009. PMID: 19492978 Review.
Cited by
- Strategies to Improve the Safety of iPSC-Derived β Cells for β Cell Replacement in Diabetes.
Pellegrini S, Zamarian V, Sordi V. Pellegrini S, et al. Transpl Int. 2022 Aug 24;35:10575. doi: 10.3389/ti.2022.10575. eCollection 2022. Transpl Int. 2022. PMID: 36090777 Free PMC article. Review. - Improved safety of induced pluripotent stem cell-derived antigen-presenting cell-based cancer immunotherapy.
Mashima H, Zhang R, Kobayashi T, Tsukamoto H, Liu T, Iwama T, Hagiya Y, Yamamoto M, Fukushima S, Okada S, Idiris A, Kaneko S, Nakatsura T, Ohdan H, Uemura Y. Mashima H, et al. Mol Ther Methods Clin Dev. 2021 Mar 5;21:171-179. doi: 10.1016/j.omtm.2021.03.002. eCollection 2021 Jun 11. Mol Ther Methods Clin Dev. 2021. PMID: 33816647 Free PMC article. - Derivation and applications of human hepatocyte-like cells.
Li S, Huang SQ, Zhao YX, Ding YJ, Ma DJ, Ding QR. Li S, et al. World J Stem Cells. 2019 Aug 26;11(8):535-547. doi: 10.4252/wjsc.v11.i8.535. World J Stem Cells. 2019. PMID: 31523372 Free PMC article. Review. - Increased Cytotoxicity of Herpes Simplex Virus Thymidine Kinase Expression in Human Induced Pluripotent Stem Cells.
Iwasawa C, Tamura R, Sugiura Y, Suzuki S, Kuzumaki N, Narita M, Suematsu M, Nakamura M, Yoshida K, Toda M, Okano H, Miyoshi H. Iwasawa C, et al. Int J Mol Sci. 2019 Feb 14;20(4):810. doi: 10.3390/ijms20040810. Int J Mol Sci. 2019. PMID: 30769780 Free PMC article. - Stem cells and their potential clinical applications in psychiatric disorders.
Ratajczak MZ, Ciechanowicz AK, Kucharska-Mazur J, Samochowiec J. Ratajczak MZ, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2018 Jan 3;80(Pt A):3-9. doi: 10.1016/j.pnpbp.2017.04.020. Epub 2017 Apr 20. Prog Neuropsychopharmacol Biol Psychiatry. 2018. PMID: 28435007 Free PMC article. Review.
References
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. - DOI - PMC - PubMed
- Aractingi S, Kanitakis J, Euvrard S, Le DC, Peguillet I, Khosrotehrani K, Lantz O, Carosella ED. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res. 2005;65:1755–1760. - PubMed
- Arafat WO, Gomez-Navarro J, Xiang J, Barnes MN, Mahasreshti P, Alvarez RD, Siegal GP, Badib AO, Buchsbaum D, Curiel DT, Stackhouse MA. An adenovirus encoding proapoptotic Bax induces apoptosis and enhances the radiation effect in human ovarian cancer. Mol Ther. 2000;1:545–554. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 HL66952/HL/NHLBI NIH HHS/United States
- U01 HL066952/HL/NHLBI NIH HHS/United States
- U01 HL066952-05/HL/NHLBI NIH HHS/United States
- U01 NS047458-03/NS/NINDS NIH HHS/United States
- U01 NS047458/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources