Molecular regulation of tumor angiogenesis and perfusion via redox signaling - PubMed (original) (raw)
Review
Molecular regulation of tumor angiogenesis and perfusion via redox signaling
Thomas W Miller et al. Chem Rev. 2009 Jul.
No abstract available
Figures
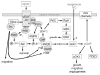
Figure 1
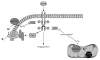
VEGF binding to VEGFR2 on endothelial cells activates its Tyr kinase activity and results in autophosphorylation of the receptor at several cytoplasmic sites. The phosphorylated Tyr serve as docking sites for specific signaling molecules. Phosphatidyinositol 3-kinase (PI3K) is recruited at phosphorylated Tyr801, increasing inositol 1,4,5-triphosphate (IP3) formation, which in turn activates 3-phosphoinositide-dependent protein kinase-1 (PDK1) to phosphorylate and activate Akt, which phosphorylates human eNOS at Ser117712,13. This phosphorylation activates eNOS and decreases its calcium dependence. VEGFR2 phosphorylation at Tyr951 recruits TSAd and Src, which phosphorylates heat shock protein 90 (Hsp90) at Tyr, which induces Hsp90 binding to eNOS and activation of NO synthesis , and phosphorylates eNOS at Tyr, which is also required for eNOS activation . Phosphorylation of VEGFR2 at Tyr1175 recruits phospholipase-Cγ (PLCγ), which mobilizes intracellular Ca2+ and thereby further activates eNOS in a calmodulin (CaM)-dependent manner. PLCγ also increases AMP kinase (AMPK)-mediated eNOS phosphorylation at Ser117788. NO produced by eNOS binds to the heme on soluble guanylate cyclase (sGC) to stimulate cGMP synthesis. cGMP in turn activates cGMP-dependent protein kinase (cGK-I) and cGMP-gated channels to regulate downstream targets that increase endothelial cell proliferation, migration, survival, and permeability. cGMP also binds to and regulates several phosphodiesterases that terminate that cGMP signal or mediate cross talk with cAMP signaling by hydrolyzing that second messenger. Additional parallel signaling through Src, Akt, and the protein kinase C-mitogen-activated protein kinase pathway (PKC-Raf1-MEK-ERK) synergizes with NO/cGMP signaling to support each of these endothelial cell responses.
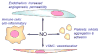
Figure 2
Nitric oxide acts on several target cells in blood vessels that are important for tumor biology. NO acts in endothelial cells to stimulate angiogenesis and increase vascular permeability. The former is important to neovascularization to support tumor growth. The latter contributes to the characteristic leakiness of the tumor vasculature. NO stimulates proliferation and migration of VSMC, which can contribute to angiogenesis. NO also relaxes VSMC, which can either increase or decrease tumor perfusion. NO inhibits platelet adhesion and aggregation. Platelet adhesion to circulating tumor cells plays an important role in metastatic spread of cancers. Finally, NO has inhibitory activities for immune cells, which can limit host anti-tumor immunity.
Figure 3
Thrombospondin-1 (TSP1) binds to two receptors on endothelial cells that inhibit NO signaling. The second and third central type 1 repeats of TSP1 contain sequences with known CD36-binding activities. The intact protein, recombinant type 1 repeats or synthetic peptides derived from the active repeats bind to CD36 and inhibit uptake of myristate via this plasma membrane fatty acid translocase. Myristate mediates membrane anchoring of a number of signaling proteins including the Src kinases. Membrane anchoring of Src and other undefined targets leads to increased activation of eNOS, which is inhibited by TSP1 . Simultaneously, myristate activates eNOS in an AMP kinase (AMPK)-dependent manner , which may also be inhibited by TSP1. Inhibition of NO signaling via CD36 requires concentrations of TSP1 that exceed those normally circulating in plasma but can be found in plasma of some cancer patients,,. The more potent TSP1 inhibitory pathway involves the signaling receptor CD47. Two CD47-binding sequences have been identified in the C-terminal domain of TSP1. Engaging CD47 signals through undefined pathways that simultaneously inhibit activation of soluble guanylate cyclase (sGC) and cGMP-dependent protein kinase (cGK-I),. These pathways inhibit NO signaling due either endogenous or exogenous NO.
Figure 4
The NO/cGMP pathway is a signaling node for pro- and anti-angiogenic signaling. In addition to VEGF, angiopoietin-1, estrogens, and insulin can activate eNOS to increase NO synthesis in endothelial cells. TSP1 signaling via CD36 and CD47 inhibits downstream elements of this pathway. Oxidized LDL (oxLDL) is another known ligand of CD36 that could inhibit angiogenesis via an overlapping pathway. In addition, oxLDL signaling inhibits protein kinase Cα, which phosphorylates eNOS at Thr495 and activates NADPH oxidase (NOX), and the resulting superoxide depletes NO levels. The angiogenesis inhibitors endostatin and vasoinhibins activate the phosphatase PP2A, which dephosphorylates and thereby inactivates eNOS.
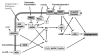
Figure 5
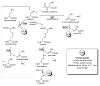
VEGF signaling leads to H2O2 production and positive feedback through inhibition of PTPs. VEGF binding to its receptor (VEGFR) results in autophosphorylation of tyrosine residues leading to downstream kinase activation and angiogenic activity. VEGFR activation also leads to activation of the small GTPase RAC1 which, in conjunction with other cytosolic components (gp47- and p67phox), activates NADPH oxidase. NADPH oxidase reduces dioxygen to superoxide subsequently converted to H2O2 by SODs. H2O2 either directly or through a thiol peroxidase intermediate inactivates PTPs which oppose the actions of VEGFR thus enhancing and sustaining the downstream signal. Another source of superoxide is from the reduction of oxygen by the uncoupling of the mitochondrial electron transport chain (through complex -I or –III). This may contribute to the potentiation of VEGFR signaling as well.
Figure 6
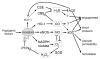
Superoxide and hydrogen peroxide reactivity. Superoxide generated by the mitochondria and NADPH oxidase has three main cellular fates; reaction with NO to form superoxide, reaction with iron-sulfur cluster (Fe-S) proteins to release ferric iron and hydroxyl radical, and reaction with super oxide dismutases (SOD) to form hydrogen peroxide. Hydrogen peroxide is consumed in scavenging and signaling reactions over a wide range of second order rate constants. References: NO; [Fe-S], SOD, catalase,, GPx, Prx, Trx, PTP, GSH.
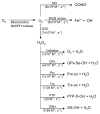
Figure 7
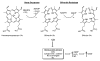
CO biosynthesis. Carbon monoxide is synthesized by the heme oxygenases (HOs). HOs bind heme and use it to activate oxygen to catalyze its own oxidation to biliverdin and CO. biliverdin is subsequently reduced by biliverdin reductase to bilirubin. CO signaling is exclusively due to interaction with reduced heme proteins including those that are targets of NO and O2.
Figure 8
H2S bio synthesis. H2S is synthesized by 3 different enzymes: cystathionine beta-synthase (CBS), cystathionine γ-lyase (CSE), and mercaptopyruvate sulfurtransferase (MPST). CBS condense cysteine and homocysteine to make cystathionine and H2S. CSE takes cystine and converts it to thiocysteine, pyruvate, and ammonia. Thiocysteine can be reduced by another equivalent of thiol to cysteine and H2S. Cysteine, converted in the mitochondria by aspartate aminotransferase (AAT) to 3-mercaptopyruvate, is converted to H2S, pyruvate, and ammonia by MPST.
Figure 9
Cross talk between H2S, CO, NO, O2•−, and H2O2 in vascular angiogenic signaling. Binding of an angiogenic factor to its cell surface receptor results in receptor tyrosine phosphorylation and downstream kinase activation of HO-1 (producing CO), eNOS (producing NO), and NADPH oxidase (producing O2•−). Receptor activation can also increase intracellular calcium levels activating CSE (producing H2S). O2•− is converted to H2O2 by SOD and this is capable of either direct or indirect inhibition of PTPs that normally attenuate receptor downstream signaling. H2O2 provides a reinforcement of the angiogenic signal. O2•− can also react with and consume NO making OONO− decreasing the signaling capacity of both NO and O2•−. NO through sGC is a primary regulator of angiogenesis, blood pressure, vascular permeability, and hemostasis. Likewise CO can bind sGC to regulate the same processes, but with a much lower affinity (pM vs. mM). However, CO also regulates angiogenic signaling in a sGC independent manner. H2S is an inhibitor of ACE with associated effects on blood pressure and has an ill-defined role in angiogenesis. Increase in CO by HO-1 induction could also decrease the levels of H2S derived from CBS.
Similar articles
- Catecholamines regulate tumor angiogenesis.
Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Chakroborty D, et al. Cancer Res. 2009 May 1;69(9):3727-30. doi: 10.1158/0008-5472.CAN-08-4289. Epub 2009 Apr 21. Cancer Res. 2009. PMID: 19383906 Free PMC article. Review. - Redundant angiogenic signaling and tumor drug resistance.
Gacche RN, Assaraf YG. Gacche RN, et al. Drug Resist Updat. 2018 Jan;36:47-76. doi: 10.1016/j.drup.2018.01.002. Epub 2018 Jan 17. Drug Resist Updat. 2018. PMID: 29499837 Review. - Why haven't we cured cancer?
Harding E. Harding E. Lancet Oncol. 2017 Jul;18(7):861-862. doi: 10.1016/S1470-2045(17)30479-5. Lancet Oncol. 2017. PMID: 28677566 No abstract available. - Editorial: Angiogenesis agents.
Marcinkiewicz C. Marcinkiewicz C. Curr Pharm Des. 2007;13(35):3543-4. doi: 10.2174/138161207782794112. Curr Pharm Des. 2007. PMID: 18220790 No abstract available. - Classical and non-classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment.
Marech I, Leporini C, Ammendola M, Porcelli M, Gadaleta CD, Russo E, De Sarro G, Ranieri G. Marech I, et al. Cancer Lett. 2016 Sep 28;380(1):216-26. doi: 10.1016/j.canlet.2015.07.028. Epub 2015 Jul 31. Cancer Lett. 2016. PMID: 26238184 Review.
Cited by
- Activated PyK2 and Its Associated Molecules Transduce Cellular Signaling from the Cancerous Milieu for Cancer Metastasis.
Lee D, Hong JH. Lee D, et al. Int J Mol Sci. 2022 Dec 7;23(24):15475. doi: 10.3390/ijms232415475. Int J Mol Sci. 2022. PMID: 36555115 Free PMC article. Review. - Monitoring the dynamics of hemeoxygenase-1 activation in head and neck cancer cells in real-time using plasmonically enhanced Raman spectroscopy.
Panikkanvalappil SR, Garlapati C, Hooshmand N, Aneja R, El-Sayed MA. Panikkanvalappil SR, et al. Chem Sci. 2019 Mar 22;10(18):4876-4882. doi: 10.1039/c9sc00093c. eCollection 2019 May 14. Chem Sci. 2019. PMID: 31183038 Free PMC article. - Modulation of Nrf2/HO-1 by Natural Compounds in Lung Cancer.
Ghareghomi S, Moosavi-Movahedi F, Saso L, Habibi-Rezaei M, Khatibi A, Hong J, Moosavi-Movahedi AA. Ghareghomi S, et al. Antioxidants (Basel). 2023 Mar 16;12(3):735. doi: 10.3390/antiox12030735. Antioxidants (Basel). 2023. PMID: 36978983 Free PMC article. Review. - Redox regulation of SERCA2 is required for vascular endothelial growth factor-induced signaling and endothelial cell migration.
Evangelista AM, Thompson MD, Weisbrod RM, Pimental DR, Tong X, Bolotina VM, Cohen RA. Evangelista AM, et al. Antioxid Redox Signal. 2012 Oct 15;17(8):1099-108. doi: 10.1089/ars.2011.4022. Epub 2012 May 31. Antioxid Redox Signal. 2012. PMID: 22472004 Free PMC article. - A NIR-Activated and Mild-Temperature-Sensitive Nanoplatform with an HSP90 Inhibitor for Combinatory Chemotherapy and Mild Photothermal Therapy in Cancel Cells.
Peng Y, Jiang H, Li B, Liu Y, Guo B, Gan W. Peng Y, et al. Pharmaceutics. 2023 Aug 31;15(9):2252. doi: 10.3390/pharmaceutics15092252. Pharmaceutics. 2023. PMID: 37765221 Free PMC article.
References
- Black WC, Welch HG. N Engl J Med. 1993;328:1237. - PubMed
- Folkman J. N Engl J Med. 1971;285:1182. - PubMed
- Naumov GN, Folkman J, Straume O, Akslen LA, editors. APMIS. Vol. 116. 2008. p. 569. - PubMed
- Beecken WD, Engl T, Ringel EM, Camphausen K, Michaelis M, Jonas D, Folkman J, Shing Y, Blaheta RA. Ann Surg Oncol. 2006;13:1241. - PubMed
- Lien S, Lowman HB. Handb Exp Pharmacol. 2008;181:131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources