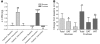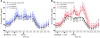Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans - PubMed (original) (raw)
. 2009 May;119(5):1322-34.
doi: 10.1172/JCI37385. Epub 2009 Apr 20.
Jean Marc Schwarz, Nancy L Keim, Steven C Griffen, Andrew A Bremer, James L Graham, Bonnie Hatcher, Chad L Cox, Artem Dyachenko, Wei Zhang, John P McGahan, Anthony Seibert, Ronald M Krauss, Sally Chiu, Ernst J Schaefer, Masumi Ai, Seiko Otokozawa, Katsuyuki Nakajima, Takamitsu Nakano, Carine Beysen, Marc K Hellerstein, Lars Berglund, Peter J Havel
Affiliations
- PMID: 19381015
- PMCID: PMC2673878
- DOI: 10.1172/JCI37385
Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans
Kimber L Stanhope et al. J Clin Invest. 2009 May.
Abstract
Studies in animals have documented that, compared with glucose, dietary fructose induces dyslipidemia and insulin resistance. To assess the relative effects of these dietary sugars during sustained consumption in humans, overweight and obese subjects consumed glucose- or fructose-sweetened beverages providing 25% of energy requirements for 10 weeks. Although both groups exhibited similar weight gain during the intervention, visceral adipose volume was significantly increased only in subjects consuming fructose. Fasting plasma triglyceride concentrations increased by approximately 10% during 10 weeks of glucose consumption but not after fructose consumption. In contrast, hepatic de novo lipogenesis (DNL) and the 23-hour postprandial triglyceride AUC were increased specifically during fructose consumption. Similarly, markers of altered lipid metabolism and lipoprotein remodeling, including fasting apoB, LDL, small dense LDL, oxidized LDL, and postprandial concentrations of remnant-like particle-triglyceride and -cholesterol significantly increased during fructose but not glucose consumption. In addition, fasting plasma glucose and insulin levels increased and insulin sensitivity decreased in subjects consuming fructose but not in those consuming glucose. These data suggest that dietary fructose specifically increases DNL, promotes dyslipidemia, decreases insulin sensitivity, and increases visceral adiposity in overweight/obese adults.
Figures
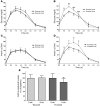
Figure 1. Changes of BW and abdominal fat.
(A) Changes of BW during the 2-week inpatient baseline, 8-week outpatient intervention, and 2-week inpatient intervention periods. **P < 0.01; ****P < 0.0001, day 56 outpatient:intervention vs. day 1 outpatient:intervention; paired Student’s t test. Glucose, n = 15; fructose, n = 17. (B) Changes of total abdominal adipose tissue, SAT, and VAT volume in subjects after consuming glucose- or fructose-sweetened beverages for 10 weeks. *P < 0.05; **P < 0.01, 10 weeks vs. 0 weeks; paired Student’s t test. Glucose, n = 14; fructose, n = 17. Data represent mean ± SEM.
Figure 2. Plasma TG.
24-hour circulating TG concentrations in subjects before and after 2, 8, and 10 weeks of consuming glucose-sweetened beverages (A) or fructose-sweetened beverages (B). ++P < 0.01 PROC MIXED 3-factor RM ANOVA with prior day’s energy intake covariable for 23-hour TG AUC. Glucose, n = 14; fructose, n = 17. Data represent mean ± SEM.
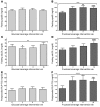
Figure 3. apoB, sdLDL, and RLP-TG.
Fasting apoB concentrations in subjects before and after 2, 8, and 10 weeks of consuming glucose-sweetened beverages (A) or fructose-sweetened beverages (B). Fasting sdLDL concentrations in subjects before and after 2, 8, and 10 weeks of consuming glucose-sweetened beverages (C) or fructose-sweetened beverages (D). Postprandial RLP-TG concentrations in subjects before and after 2, 8, and 10 weeks of consuming glucose-sweetened beverages (E) or fructose-sweetened beverages (F). ++P < 0.01; +++P < 0.001; ++++P < 0.0001, PROC MIXED 3-factor RM ANOVA (C and D) with prior day’s energy intake covariable (A, B, E, and F). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, Tukey’s multiple comparison test vs. 0 weeks. Glucose, n = 15; fructose, n = 17. Data represent mean ± SEM.
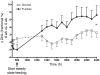
Figure 4. Hepatic fractional DNL.
Change of fractional DNL before and during steady-state feeding of meals with glucose- or fructose-sweetened beverages (9 weeks) compared with high–complex carbohydrate meals (0 weeks). *P = 0.016, GLM ANOVA, effect of sugar on Δ of 16-hour fractional DNL AUC at 9 weeks vs. 0 weeks. Glucose, n = 8; fructose, n = 10. Data represent mean ± SEM.
Figure 5. OGTT and glucose disposal test.
Glucose concentrations during an OGTT in subjects before and after 9 weeks of consuming (A) glucose-sweetened beverages or (B) fructose-sweetened beverages. Insulin concentrations during an OGTT in subjects before and after 9 weeks of consuming glucose-sweetened beverages (C) or fructose-sweetened beverages (D). *P < 0.05; **P < 0.01; ***P < 0.001, paired Student’s t test, 9 weeks vs. 0 weeks. Glucose, n = 15; fructose, n = 17. Insulin sensitivity index during glucose disposal test as percentage of baseline in subjects before and after 9 weeks of consuming glucose- or fructose-sweetened beverages (E). **P < 0.01, paired Student’s t test, 9 weeks vs. 0 weeks. Glucose: n = 14; fructose: n = 17. Data represent mean ± SEM.
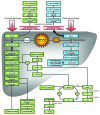
Figure 6. Proposed mechanisms underlying the differential effects of fructose and glucose consumption.
Hepatic glucose metabolism is regulated by phosphofructokinase, which is inhibited by ATP and citrate when energy status is high, thus limiting hepatic uptake of dietary glucose and production of DNL substrates. The hepatic metabolism of dietary fructose is independent of energy status, resulting in unregulated hepatic fructose uptake and increased lipogenesis. The resulting increased hepatic lipid decreases apoB degradation and increases production/secretion of VLDL-TG, mainly as TG-rich VLDL1 (29). This, along with chylomicron competition for LPL-mediated TG hydrolysis and reduced LPL activation by insulin, results in longer VLDL residence time, allowing for augmented cholesteryl ester transfer protein–mediated (CETP-mediated) lipid exchanges with LDL and increased LDL-TG and RLP levels. Hydrolysis of LDL-TG by hepatic lipase increases plasma sdLDL concentrations. After an overnight fast, DNL is no longer elevated and VLDL and chylomicrons remnants have been cleared; thus, plasma TG levels are normal. Postprandially, the increment of plasma apoB levels is associated with VLDL particles; in the fasting state, it is presumably associated with sdLDL, which turns over more slowly. As SAT is more sensitive to insulin activation of LPL activity than VAT, reduced postmeal insulin exposure may lead to less TG uptake in SAT and thus increased TG uptake/accumulation in VAT. Increased hepatic lipid supply may also induce hepatic insulin resistance, possibly through increased levels of diacylglycerol, which activates novel PKC (85). Novel PKC decreases tyrosine phosphorylation of the insulin receptor/insulin receptor substrate 1, resulting in increased hepatic glucose production, impaired glucose tolerance, and increased fasting glucose and insulin concentrations. oxLDL, oxidized LDL.
Comment in
- Dietary sugars: a fat difference.
Hofmann SM, Tschöp MH. Hofmann SM, et al. J Clin Invest. 2009 May;119(5):1089-92. doi: 10.1172/jci39332. J Clin Invest. 2009. PMID: 19422101 Free PMC article.
Similar articles
- Effects of sugar-sweetened beverages on plasma acylation stimulating protein, leptin and adiponectin: relationships with metabolic outcomes.
Rezvani R, Cianflone K, McGahan JP, Berglund L, Bremer AA, Keim NL, Griffen SC, Havel PJ, Stanhope KL. Rezvani R, et al. Obesity (Silver Spring). 2013 Dec;21(12):2471-80. doi: 10.1002/oby.20437. Epub 2013 Jun 13. Obesity (Silver Spring). 2013. PMID: 23512943 Free PMC article. - Dietary sugars: a fat difference.
Hofmann SM, Tschöp MH. Hofmann SM, et al. J Clin Invest. 2009 May;119(5):1089-92. doi: 10.1172/jci39332. J Clin Invest. 2009. PMID: 19422101 Free PMC article. - Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses.
Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Teff KL, et al. J Clin Endocrinol Metab. 2009 May;94(5):1562-9. doi: 10.1210/jc.2008-2192. Epub 2009 Feb 10. J Clin Endocrinol Metab. 2009. PMID: 19208729 Free PMC article. Clinical Trial. - Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup.
Stanhope KL, Havel PJ. Stanhope KL, et al. Am J Clin Nutr. 2008 Dec;88(6):1733S-1737S. doi: 10.3945/ajcn.2008.25825D. Am J Clin Nutr. 2008. PMID: 19064538 Free PMC article. Review. - Sugar consumption, metabolic disease and obesity: The state of the controversy.
Stanhope KL. Stanhope KL. Crit Rev Clin Lab Sci. 2016;53(1):52-67. doi: 10.3109/10408363.2015.1084990. Epub 2015 Sep 17. Crit Rev Clin Lab Sci. 2016. PMID: 26376619 Free PMC article. Review.
Cited by
- Effects of sugar-sweetened beverages on plasma acylation stimulating protein, leptin and adiponectin: relationships with metabolic outcomes.
Rezvani R, Cianflone K, McGahan JP, Berglund L, Bremer AA, Keim NL, Griffen SC, Havel PJ, Stanhope KL. Rezvani R, et al. Obesity (Silver Spring). 2013 Dec;21(12):2471-80. doi: 10.1002/oby.20437. Epub 2013 Jun 13. Obesity (Silver Spring). 2013. PMID: 23512943 Free PMC article. - Sugar intake, obesity, and diabetes in India.
Gulati S, Misra A. Gulati S, et al. Nutrients. 2014 Dec 22;6(12):5955-74. doi: 10.3390/nu6125955. Nutrients. 2014. PMID: 25533007 Free PMC article. Review. - Adverse effects of honey on low-density lipoprotein cholesterol and adiponectin concentrations in patients with type 2 diabetes: a randomized controlled cross-over trial.
Sadeghi F, Akhlaghi M, Salehi S. Sadeghi F, et al. J Diabetes Metab Disord. 2020 Apr 14;19(1):373-380. doi: 10.1007/s40200-020-00518-z. eCollection 2020 Jun. J Diabetes Metab Disord. 2020. PMID: 32550188 Free PMC article. - Relationship of dieting and restrained eating to self-reported caloric intake in female college freshmen.
Goldstein SP, Katterman SN, Lowe MR. Goldstein SP, et al. Eat Behav. 2013 Apr;14(2):237-40. doi: 10.1016/j.eatbeh.2012.12.002. Epub 2012 Dec 20. Eat Behav. 2013. PMID: 23557829 Free PMC article. - The good, the bad, and the unknown: Fructose and FGF21.
Hofmann SM, Havel PJ. Hofmann SM, et al. Mol Metab. 2014 Nov 14;4(1):1-2. doi: 10.1016/j.molmet.2014.11.002. eCollection 2015 Jan. Mol Metab. 2014. PMID: 25685684 Free PMC article. No abstract available.
References
- Le K.A., Tappy L. Metabolic effects of fructose. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:469–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AT-002993/AT/NCCIH NIH HHS/United States
- R21 AT002599/AT/NCCIH NIH HHS/United States
- AT-003545/AT/NCCIH NIH HHS/United States
- R21 AT002993/AT/NCCIH NIH HHS/United States
- UL1 RR024146/RR/NCRR NIH HHS/United States
- R01 HL075675/HL/NHLBI NIH HHS/United States
- R01 HL107256/HL/NHLBI NIH HHS/United States
- R01 HL091333/HL/NHLBI NIH HHS/United States
- R01 HL-075675/HL/NHLBI NIH HHS/United States
- AT-002599/AT/NCCIH NIH HHS/United States
- HL-091333/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous