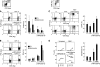Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms - PubMed (original) (raw)
Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms
Masahisa Jinushi et al. J Exp Med. 2009.
Abstract
Carcinogenesis reflects the dynamic interplay of transformed cells and normal host elements, but cancer treatments typically target each compartment separately. Within the tumor microenvironment, the secreted protein milk fat globule epidermal growth factor-8 (MFG-E8) stimulates disease progression through coordinated alpha(v)beta(3) integrin signaling in tumor and host cells. MFG-E8 enhances tumor cell survival, invasion, and angiogenesis, and contributes to local immune suppression. We show that systemic MFG-E8 blockade cooperates with cytotoxic chemotherapy, molecularly targeted therapy, and radiation therapy to induce destruction of various types of established mouse tumors. The combination treatments evoke extensive tumor cell apoptosis that is coupled to efficient dendritic cell cross-presentation of dying tumor cells. This linkage engenders potent antitumor effector T cells but inhibits FoxP3(+) T reg cells, thereby achieving long-term protective immunity. Collectively, these findings suggest that systemic MFG-E8 blockade might intensify the antitumor activities of existing therapeutic regimens through coordinated cell-autonomous and immune-mediated mechanisms.
Figures
Figure 1.
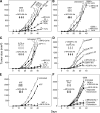
MFG-E8 antibody blockade synergizes with cytotoxic therapies to mediate tumor destruction. (A) Established MC38 carcinomas (25 mm2) were treated with systemic gemcitabine (GEM) and/or an anti–MFG-E8 mAb, as indicated. (B) Same conditions as in A, but with rabbit anti–MFG-E8 sera. (C) Established MC38 carcinomas were treated with 5-FU or CPT-11 with or without anti–MFG-E8 mAb. (D) Established MC38 carcinomas were treated with anti–VEGFR-2 mAb DC101 or EGFR-TKI AG490 with or without anti–MFG-E8 mAb. (E) Established MC38 tumors were treated with local irradiation (XRT) with or without systemic anti–MFG-E8 mAb. (F) Established B16 melanomas (25 mm2) were treated with systemic doxorubicin, etoposide, or GEM with or without anti–MFG-E8 mAb. Each experiment was performed with five mice per group, and similar results were observed for each panel in five independent experiments. Shown are the means ± SEM per cohort for a representative experiment. *, P < 0.05 between a treatment group and control.
Figure 2.
Drug-resistant tumor cells express MFG-E8. (A) MC38 carcinoma and B16 melanoma cells were treated with various cytotoxic agents under serum-free condition for 24 h, and intracellular MFG-E8 expression in viable cells (annexin V−/propidium iodide−) was determined with flow cytometry. The shaded and open histograms represent the levels of expression on untreated and treated cells, respectively. Gemcitabine (GEM) and 5-FU accomplished minimal killing of B16 cells (not depicted). (B) MFG-E8 levels in culture supernatants from A were measured with ELISA. (C) EL-4 thymoma cells were treated with γ irradiation (100 Gy), and MFG-E8 expression in viable cells (annexin V−/propidium iodide−) was determined with flow cytometry. (D) Stable drug-resistant variants of MC38 were generated and tested for MFG-E8 expression with flow cytometry (shaded histogram). The staining with isotype control antibodies is also shown. (E) MC38 carcinoma cells were exposed to GEM or 5-FU in the presence of anti–MFG-E8 mAb or isotype control as in A, and cell viability was determined with flow cytometry (percentages are shown). (F) Established MC38 and B16 tumors (25 mm2) were treated with GEM or dacarbazine, respectively, with or with systemic anti–MFG-E8 mAb as in Fig. 1. 4 d after completion of therapy, tumor homogenates were prepared and assayed for caspase 3 activation with ELISA. Presented data are representative of three independent experiments with similar results. Means ± SEM are shown in B and F. *, P < 0.05 between the treatment and control.
Figure 3.
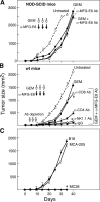
The therapeutic activity of MFG-E8 antibody blockade and chemotherapy involves host immunity. (A) NOD-SCID mice harboring established MC38 carcinomas (25 mm2) were treated with systemic GEM and anti–MFG-E8 mAb. (B) Established MC38 carcinomas bearing wild-type C57BL/6 mice that were depleted of CD4+, CD8+, or NK1.1+ cells with antibodies were treated with systemic gemcitabine (GEM) and anti–MFG-E8 mAb. (C) Wild-type C57BL/6 mice that had rejected established MC38 carcinomas with systemic GEM and anti–MFG-E8 mAb showed specific long-term protective immunity against subsequent challenge with MC38 cells during the follow-up period (>200 d). Each experiment was performed with five mice per group, and similar results were observed for each panel in three independent experiments. Shown are the means ± SEM for each cohort in a representative experiment. *, P < 0.05 between the treatment and control.
Figure 4.
Combination MFG-E8 antibody blockade and chemotherapy enhances antitumor effector T cells and inhibits FoxP3+ T reg cells. Tumor-infiltrating lymphocytes (TILs) were harvested from mice bearing MC38 tumors 5 d after the indicated treatment. The TILs were gated as CD3+CD4+ or CD3+CD8+ T cells, and assayed for (A) FoxP3, (B) IFN-γ, and (C) CD44 expression with flow cytometry (percentages are shown). Representative stainings are presented. The means ± SEM for six mice per group are shown in the adjacent panels. (D) Draining lymph nodes were harvested from MC38-bearing mice after the indicated treatments and evaluated for cytotoxic activity against 51Cr-labeled MC38 and B16 targets in vitro. The percent specific lysis is presented. Each experiment was independently performed four times. The means ± SEM at an effector/target ratio of 100:1 for six mice per group are shown in the adjacent panels. *, P < 0.05 between the treatment and control.
Figure 5.
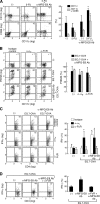
Anti–MFG-E8 antibodies enhance dendritic cell cross-presentation of dying tumor cells. (A) Tumor-infiltrating cells were harvested from mice harboring MC38 carcinomas 4 d after the indicated treatment. The CD3+ and B220+ lymphocytes were excluded by gating, and the remaining forward/side scatter high cells were analyzed for CD11c, CD11b, and CD86 with flow cytometry (percentages are shown). Similar results were observed in three experiments. Shown to the right are the means ± SEM for five mice per group. *, P < 0.05. (B) BMDCs were co-cultured with PKH26-labeled EG.7-OVA cells (with or without opsonization with anti–MFG-E8 mAbs) and evaluated for phagocytosis. The impact of blocking antibodies to αv integrins and Fc receptors was determined (percentages are shown). Similar results were observed in three experiments, and the means ± SEM are shown. *, P < 0.05. (C) BMDCs that were loaded with EG.7-OVA cells as in B were co-cultured with OVA-specific TCR transgenic CD4+ T cells, and IFN-γ production was evaluated with flow cytometry (percentages are shown). The effects of anti–αv integrin and Fc receptor antibodies are shown. Similar results were observed in three experiments, and the means ± SEM are shown to the right. *, P < 0.05. (D) 106 irradiated EG.7-OVA cells per mouse were injected into the footpads of OT-I mice with anti–MFG-E8, anti-FcγR, and isotype control antibodies as indicated. The draining lymph nodes were harvested after 5 d, and CD8+ T cell IFN-γ production was determined with flow cytometry (percentages are shown). Similar results were observed in three experiments. Shown are the means ± SEM. *, P < 0.05.
Figure 6.
Anti–MFG-E8 antibodies modulate antigen-presenting cell cytokine profiles. (A) BMDCs were treated with recombinant MFG-E8 or anti–MFG-E8 mAbs, and cytokine production was determined with ELISA. Shown are the means ± SEM for three experiments. *, P < 0.05. (B) C57BL/6 wild-type or IL-12p35–deficient mice harboring established MC38 colon carcinomas (25 mm2) were treated with systemic gemcitabine with or without anti–MFG-E8 mAb, as shown in Fig. 1 A. Shown are the means ± SEM for five mice per group. Similar results were observed in a second experiment. *, P < 0.05 between treated wild-type and IL-12 KO mice. (C) BMDCs from wild-type or IL-12–deficient mice were loaded with EG.7-OVA cells and co-cultured with CD4+ T cells obtained from wild-type mice that were immunized with EG.7-OVA cells (five times). IFN-γ production was evaluated by flow cytometry (percentages are shown). Similar results were observed in a second experiment.
Similar articles
- Down-regulation of MFG-E8 by RNA interference combined with doxorubicin triggers melanoma destruction.
Zhao JY, Ma XL, Li ZM, Deng R, Wang SM, Shen GB, Zhang J, Wang FT, Zhang BL, Wei YQ. Zhao JY, et al. Clin Exp Med. 2015 May;15(2):127-35. doi: 10.1007/s10238-014-0277-6. Epub 2014 Mar 12. Clin Exp Med. 2015. PMID: 24619299 - Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens.
Peng Y, Elkon KB. Peng Y, et al. J Clin Invest. 2011 Jun;121(6):2221-41. doi: 10.1172/JCI43254. Epub 2011 May 2. J Clin Invest. 2011. PMID: 21537078 Free PMC article. - Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment.
Jinushi M, Nakazaki Y, Carrasco DR, Draganov D, Souders N, Johnson M, Mihm MC, Dranoff G. Jinushi M, et al. Cancer Res. 2008 Nov 1;68(21):8889-98. doi: 10.1158/0008-5472.CAN-08-2147. Cancer Res. 2008. PMID: 18974133 - MFG-E8 regulates the immunogenic potential of dendritic cells primed with necrotic cell-mediated inflammatory signals.
Baghdadi M, Chiba S, Yamashina T, Yoshiyama H, Jinushi M. Baghdadi M, et al. PLoS One. 2012;7(6):e39607. doi: 10.1371/journal.pone.0039607. Epub 2012 Jun 25. PLoS One. 2012. PMID: 22761839 Free PMC article. - Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation.
Aziz M, Jacob A, Matsuda A, Wang P. Aziz M, et al. Apoptosis. 2011 Nov;16(11):1077-86. doi: 10.1007/s10495-011-0630-0. Apoptosis. 2011. PMID: 21901532 Review.
Cited by
- Cancer Stem Cells and Macrophages: Implications in Tumor Biology and Therapeutic Strategies.
Sainz B Jr, Carron E, Vallespinós M, Machado HL. Sainz B Jr, et al. Mediators Inflamm. 2016;2016:9012369. doi: 10.1155/2016/9012369. Epub 2016 Feb 14. Mediators Inflamm. 2016. PMID: 26980947 Free PMC article. Review. - Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy.
Crittenden MR, Baird J, Friedman D, Savage T, Uhde L, Alice A, Cottam B, Young K, Newell P, Nguyen C, Bambina S, Kramer G, Akporiaye E, Malecka A, Jackson A, Gough MJ. Crittenden MR, et al. Oncotarget. 2016 Nov 29;7(48):78653-78666. doi: 10.18632/oncotarget.11823. Oncotarget. 2016. PMID: 27602953 Free PMC article. - TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity.
Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. Freeman GJ, et al. Immunol Rev. 2010 May;235(1):172-89. doi: 10.1111/j.0105-2896.2010.00903.x. Immunol Rev. 2010. PMID: 20536563 Free PMC article. Review. - Targeting MFGE8 secreted by cancer-associated fibroblasts blocks angiogenesis and metastasis in esophageal squamous cell carcinoma.
Liu B, Zhang B, Qi J, Zhou H, Tan L, Huang J, Huang J, Fang X, Gong L, Luo J, Liu S, Fu L, Ling F, Ma S, Lai-Wan Kwong D, Wang X, Guan XY. Liu B, et al. Proc Natl Acad Sci U S A. 2023 Oct 17;120(42):e2307914120. doi: 10.1073/pnas.2307914120. Epub 2023 Oct 10. Proc Natl Acad Sci U S A. 2023. PMID: 37816055 Free PMC article. - Milk fat globule epidermal growth factor-factor 8 mitigates inflammation and tissue injury after hemorrhagic shock in experimental animals.
Zhang F, Shah KG, Qi L, Wu R, Barrera R, Nicastro J, Coppa GF, Wang P. Zhang F, et al. J Trauma Acute Care Surg. 2012 Apr;72(4):861-9. doi: 10.1097/TA.0b013e318249a97c. J Trauma Acute Care Surg. 2012. PMID: 22491597 Free PMC article.
References
- Dranoff G. 2004. Cytokines in cancer pathogenesis and cancer therapy.Nat. Rev. Cancer. 4:11–22 - PubMed
- de Visser K.E., Eichten A., Coussens L.M. 2006. Paradoxical roles of the immune system during cancer development.Nat. Rev. Cancer. 6:24–37 - PubMed
- Balkwill F., Charles K.A., Mantovani A. 2005. Smoldering and polarized inflammation in the initiation and promotion of malignant disease.Cancer Cell. 7:211–217 - PubMed
- Smyth M.J., Dunn G.P., Schreiber R.D. 2006. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity.Adv. Immunol. 90:1–50 - PubMed
- Karin M., Greten F.R. 2005. NF-kappaB: linking inflammation and immunity to cancer development and progression.Nat. Rev. Immunol. 5:749–759 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous