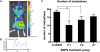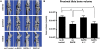The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells - PubMed (original) (raw)
The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells
Alla Bondareva et al. PLoS One. 2009.
Abstract
Lysyl oxidase (LOX), an extracellular matrix remodeling enzyme, appears to have a role in promoting breast cancer cell motility and invasiveness. In addition, increased LOX expression has been correlated with decreases in both metastases-free, and overall survival in breast cancer patients. With this background, we studied the ability of beta-aminopropionitrile (BAPN), an irreversible inhibitor of LOX, to regulate the metastatic colonization potential of the human breast cancer cell line, MDA-MB-231. BAPN was administered daily to mice starting either 1 day prior, on the same day as, or 7 days after intracardiac injection of luciferase expressing MDA-MB-231-Luc2 cells. Development of metastases was monitored by in vivo bioluminescence imaging, and tumor-induced osteolysis was assessed by micro-computed tomography (microCT). We found that BAPN administration was able to reduce the frequency of metastases. Thus, when BAPN treatment was initiated the day before, or on the same day as the intra-cardiac injection of tumor cells, the number of metastases was decreased by 44%, and 27%, and whole-body photon emission rates (reflective of total tumor burden) were diminished by 78%, and 45%, respectively. In contrast, BAPN had no effect on the growth of established metastases. Our findings suggest that LOX activity is required during extravasation and/or initial tissue colonization by circulating MDA-MB-231 cells, lending support to the idea that LOX inhibition might be useful in metastasis prevention.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. In vitro evaluation of MDA-MB-231-Luc2 cells.
(A) Cells were plated in a 96-well plate in quadruplicate, ranging from 10,000 to 2,000 cell/well. Wells with media only (no cells) were included as control. _D_-luciferin substrate was added to each well and the plate was imaged to estimate the bioluminescence of MDA-MB-231-Luc2 cells. (B) Correlation between bioluminescence and cell number per well was plotted as mean photons/s/well±SEM. (C) Fluorescence microscopy, showing that MDA-MB-231-Luc2 cells expressed EGFP. (D) 1×103 MDA-MB-231 parental and MDA-MB-231-Luc2 cells were seeded into quadruplicate wells of 96-well plates and their proliferation rates determined by MTT assay. Data was plotted as mean relative growth±SEM.
Figure 2. Growth of MDA-MB-231-Luc2 metastasis and the effects of BAPN treatment.
Bioluminescence imaging was performed 2 times per week starting on day 7 after intracardiac injection of tumor cells. Representative dorsal images of control and BAPN-treated mice are shown from 7 to 21 d post-injection (A). Images for days 7–10, and days 14 to 21 are shown with different sensitivity color scale bars, reflecting the rapid growth rate of metastases. Scales are in photons/s/cm2. Whole body luminescence, a measure of tumor burden, for the different groups is shown as a function of time (B); note the need for a log scale owing to the rapid growth of the tumors. BAPN treatment was initiated at the indicated times and then continued daily thereafter until day 21. (C) Whole body bioluminescence at day 7 post-cell injection was quantified as mean photons/s±SEM. Whole body tumor bioluminescence at day 21 (D) was quantified as mean photons/s±SEM. For both (B) and (C), n = 10 for each of ‘no BAPN’ and for ‘BAPN d 7’ groups, and n = 5 for ‘BAPN d −1’ and for ‘BAPN d 0’ groups. Asterisks indicate statistical significance (* P<0.05;** P<0.01; *** P<0.001).
Figure 3. Effect of BAPN administration on the number of metastases.
Metastases were quantified by moving a line for measuring photon emissions over the entire mouse image (A), and using this to count the number of bioluminescent peaks in the whole animal profile (B). Dorsal, ventral, and lateral images taken at day 21 after cell injection were evaluated for mice that received BAPN starting at the indicated time points, and continued daily thereafter until day 21, as well as for control (vehicle only) mice. Total number of metastases per mouse are shown as the mean±SEM (C) (n = 10 for each of ‘no BAPN’ and for ‘BAPN d 7’ groups, and n = 5 for ‘BAPN d −1’ and for ‘BAPN d 0’ groups). Asterisks indicate statistical significance (* P<0.05; ** P<0.01).
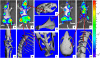
Figure 4. Correlation between tumor bioluminescence and osteolytic lesions.
Representative bioluminescence images (A, B, E, F) and corresponding 3D μCT reconstructions for some of the most common skeletal sites of bone metastases in NIH-III mice following intracardiac injection of MDA-MB-231-Luc2 cells: jaw (C), scapula (D), knee (G), ribs (H), pubis/ischium (I) parietal bone of the skull (J), and thoracic spine (K). Scale bar on the right of images represents intensity of bioluminescence in photons/s/cm2.
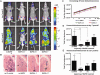
Figure 5. Development of MDA-MB-231-Luc2 metastases in the knees of mice receiving BAPN as compared to controls.
Representative ventral bioluminescence images of control and BAPN-treated mice are shown for the days 7 and 21 following injection of cells (A) with circles over the knees illustrating the placement of the regions of interest (ROI). (B) H&E stained sections showing the growth plate (GP), cortical bone (CB), trabecular bone (TB), bone marrow (BM), and tumor area (T) of representative right femurs; the scale bar is 50 µm. (C) Knee bioluminescence increases over time; at day 7 (D), and day 21 (E) after cell injection. Signals were quantified from dorsal and ventral knee ROI and graphed as mean photons/s±SEM (n = 10 for each of ‘no BAPN’ and for ‘BAPN d 7’ groups, and n = 5 for ‘BAPN d −1’ and for ‘BAPN d 0’ groups). Asterisks indicate statistical significance (* P<0.05; ** P<0.01; *** P<0.001).
Figure 6. Evaluation of knee osteolysis by μCT.
Mice undergoing BAPN treatment and their controls were sacrificed at day 21 after intracardiac injection of MDA-MB-231-Luc2 cells, and their left hind limbs were examined by μCT. Hind limbs from age-matched non-tumor cell-injected mice were used as controls. Anterior, posterior, and medial μCT images of representative knee joints from the different groups are shown (A). Bone volumes were determined from measurements of three hundred 10 µm slices taken just distal to the proximal tibial growth plate and graphed as mean bone volume±SEM (B) (n = 8 for ‘no IC’, that is, control mice that were not injected with tumor cells; n = 9 for ‘no BAPN’; n = 10 for ‘BAPN d 7’; and n = 5 for ‘BAPN d −1’ groups). Asterisk indicates a statistical significance of P<0.05.
Similar articles
- A molecular role for lysyl oxidase in breast cancer invasion.
Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ. Kirschmann DA, et al. Cancer Res. 2002 Aug 1;62(15):4478-83. Cancer Res. 2002. PMID: 12154058 - The lysyl oxidase inhibitor β-aminopropionitrile reduces body weight gain and improves the metabolic profile in diet-induced obesity in rats.
Miana M, Galán M, Martínez-Martínez E, Varona S, Jurado-López R, Bausa-Miranda B, Antequera A, Luaces M, Martínez-González J, Rodríguez C, Cachofeiro V. Miana M, et al. Dis Model Mech. 2015 Jun;8(6):543-51. doi: 10.1242/dmm.020107. Epub 2015 Apr 7. Dis Model Mech. 2015. PMID: 26035864 Free PMC article. - Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway.
Chen LC, Tu SH, Huang CS, Chen CS, Ho CT, Lin HW, Lee CH, Chang HW, Chang CH, Wu CH, Lee WS, Ho YS. Chen LC, et al. Breast Cancer Res Treat. 2012 Aug;134(3):989-1004. doi: 10.1007/s10549-012-1986-8. Epub 2012 Mar 21. Breast Cancer Res Treat. 2012. PMID: 22434522 - LOXL2 Inhibitors and Breast Cancer Progression.
Ferreira S, Saraiva N, Rijo P, Fernandes AS. Ferreira S, et al. Antioxidants (Basel). 2021 Feb 19;10(2):312. doi: 10.3390/antiox10020312. Antioxidants (Basel). 2021. PMID: 33669630 Free PMC article. Review. - Lysyl oxidase mediates hypoxic control of metastasis.
Erler JT, Giaccia AJ. Erler JT, et al. Cancer Res. 2006 Nov 1;66(21):10238-41. doi: 10.1158/0008-5472.CAN-06-3197. Cancer Res. 2006. PMID: 17079439 Review.
Cited by
- Characterization of a murine model of metastatic human non-small cell lung cancer and effect of CXCR4 inhibition on the growth of metastases.
Singla AK, Downey CM, Bebb GD, Jirik FR. Singla AK, et al. Oncoscience. 2015 Feb 9;2(3):263-71. doi: 10.18632/oncoscience.117. eCollection 2015. Oncoscience. 2015. PMID: 25897429 Free PMC article. - A framework for the development of effective anti-metastatic agents.
Anderson RL, Balasas T, Callaghan J, Coombes RC, Evans J, Hall JA, Kinrade S, Jones D, Jones PS, Jones R, Marshall JF, Panico MB, Shaw JA, Steeg PS, Sullivan M, Tong W, Westwell AD, Ritchie JWA; Cancer Research UK and Cancer Therapeutics CRC Australia Metastasis Working Group. Anderson RL, et al. Nat Rev Clin Oncol. 2019 Mar;16(3):185-204. doi: 10.1038/s41571-018-0134-8. Nat Rev Clin Oncol. 2019. PMID: 30514977 Free PMC article. - Involvement of lysyl oxidase in the pathogenesis of arterial stiffness in chronic kidney disease.
Sharma RK, Kamble SH, Krishnan S, Gomes J, To B, Li S, Liu IC, Gumz ML, Mohandas R. Sharma RK, et al. Am J Physiol Renal Physiol. 2023 Apr 1;324(4):F364-F373. doi: 10.1152/ajprenal.00239.2022. Epub 2023 Feb 24. Am J Physiol Renal Physiol. 2023. PMID: 36825626 Free PMC article. - Matrix crosslinking forces tumor progression by enhancing integrin signaling.
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Levental KR, et al. Cell. 2009 Nov 25;139(5):891-906. doi: 10.1016/j.cell.2009.10.027. Cell. 2009. PMID: 19931152 Free PMC article. - Lysyl oxidase inhibition enhances browning of white adipose tissue and adaptive thermogenesis.
Xing C, Jiang D, Liu Y, Tang Q, Huang H. Xing C, et al. Genes Dis. 2020 Oct 10;9(1):140-150. doi: 10.1016/j.gendis.2020.10.001. eCollection 2022 Jan. Genes Dis. 2020. PMID: 35005114 Free PMC article.
References
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. - PubMed
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
- Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12:153–162. - PubMed
- Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous