Lysyl oxidase (lox) gene deficiency affects osteoblastic phenotype - PubMed (original) (raw)
doi: 10.1007/s00223-009-9252-8. Epub 2009 May 21.
J M Mäki, P Weisshaupt, N Heng, A H Palamakumbura, P N'Guessan, A Ding, R Radlanski, H Renz, T A L J J Bronckers, J Myllyharju, A M Kielbassa, B M Kleber, J-P Bernimoulin, P C Trackman
Affiliations
- PMID: 19458888
- PMCID: PMC2827261
- DOI: 10.1007/s00223-009-9252-8
Lysyl oxidase (lox) gene deficiency affects osteoblastic phenotype
N Pischon et al. Calcif Tissue Int. 2009 Aug.
Abstract
Lysyl oxidase (LOX) catalyzes cross-linking of elastin and collagen, which is essential for the structural integrity and function of bone tissue. The present study examined the role of Lox gene deficiency for the osteoblast phenotype in primary calvarial osteoblasts from E18.5 Lox knockout (Lox ( -/- )) and wild type (wt) (C57BL/6) mice. Next to Lox gene depletion, mRNA expression of Lox isoforms, LOXL1-4, was significantly downregulated in Lox ( -/- ) bone tissue. A significant decrease of DNA synthesis of Lox ( -/- ) osteoblasts compared to wt was found. Early stages of osteoblastic apoptosis studied by annexin-V binding as well as later stages of DNA fragmentation were not affected. However, mineral nodule formation and osteoblastic differentiation were markedly decreased, as revealed by significant downregulation of osteoblastic markers, type I collagen, bone sialoprotein, and Runx2/Cbfa1.
Figures
Figure 1
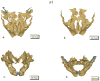
Lateral views of E 18.5 calcified tissues of _Lox_-/- and wt mice stained with Alizarin Red and Alcian Blue. Experiments were performed three times. Representative images of one experiment are shown.
Figure 2
Three-dimensional reconstructions of of _Lox_-/- and wt alveolar as well as of teeth-related structures. Reconstructions were performed two times for each genotype. Representative images (coronal (a-b), frontal (c) and dorsal (d) views) of one experiment are shown. Bone tissue is presented in brown, while Meckel's cartilage and teeth are given in blue and white colors, respectively.
Figure 2
Three-dimensional reconstructions of of _Lox_-/- and wt alveolar as well as of teeth-related structures. Reconstructions were performed two times for each genotype. Representative images (coronal (a-b), frontal (c) and dorsal (d) views) of one experiment are shown. Bone tissue is presented in brown, while Meckel's cartilage and teeth are given in blue and white colors, respectively.
Figure 3
Electronmicroscopic analysis of collagen fibril diameters of craniofacial bone tissues. For each genotype, 500 fibril diameters were measured in randomly chosen areas. Representative images are shown for _Lox_-/- and wt. (bar = 30 nm; magnification = × 35,000)
Figure 4
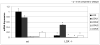
Real-time RT-PCR analysis of LOX and its isoforms, LOXL1-4, from _Lox_-/- and wt mice. Data are presented as mean ± SD obtained from three measurents of pooled RNA samples. Asterisks indicate significant differences between _Lox_-/- and wt mice (p<0.05; unpaired student's _t_-test assuming equal variances).
Figure 5
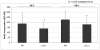
BrdU incorporation in primary _Lox_-/- and wt osteoblastic cells after 24 h and 48 h measured by ELISA at 405 nm. Data are presented as means ± SD of three experiments (n = 6 cultures/genotype). Asterisks indicate significant differences between _Lox_-/- and wt cells (p<0.05; unpaired student's _t_-test assuming equal variances).
Figure 6
Annexin-V positive (a), propidium iodine (PI) positive (b) cell count in primary _Lox_-/- and wt osteoblastic cells after 6 h, 16 h and 24 h measured by fluorescence microscopy. Measurement of DNA fragmentation (c) in primary _Lox_-/- and wt osteoblastic cells after 6 h, 16 h and 24 h by ELISA at 405 nm. Spectrophotometric measurement of LDH release (d) in primary _Lox_-/- and wt osteoblastic cells after 6 h, 16 h and 24 h at 490 nm. Data are presented as means ± SD of three experiments (n = 3 cultures/genotype).
Figure 7
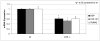
Real-time RT-PCR analysis of bone markers, COL1A1, bone sialoprotein (BSP) and Runx2/Cbfa1from _Lox_-/- and wt mice. Data are presented as mean ± SD obtained from three measurents of pooled RNA samples. Asterisks indicate significant differences between _Lox_-/- and wt cells (p<0.05; unpaired student's _t_-test assuming equal variances).
Similar articles
- Lysyl oxidase modulates the osteoblast differentiation of primary mouse calvaria cells.
Sharma-Bhandari A, Park SH, Kim JY, Oh J, Kim Y. Sharma-Bhandari A, et al. Int J Mol Med. 2015 Dec;36(6):1664-70. doi: 10.3892/ijmm.2015.2384. Epub 2015 Oct 21. Int J Mol Med. 2015. PMID: 26497171 - Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts.
Vora SR, Palamakumbura AH, Mitsi M, Guo Y, Pischon N, Nugent MA, Trackman PC. Vora SR, et al. J Biol Chem. 2010 Mar 5;285(10):7384-93. doi: 10.1074/jbc.M109.033597. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048148 Free PMC article. - Differential expression of lysyl oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta.
Behmoaras J, Slove S, Seve S, Vranckx R, Sommer P, Jacob MP. Behmoaras J, et al. Rejuvenation Res. 2008 Oct;11(5):883-9. doi: 10.1089/rej.2008.0760. Rejuvenation Res. 2008. PMID: 18803461 - Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects.
López B, González A, Hermida N, Valencia F, de Teresa E, Díez J. López B, et al. Am J Physiol Heart Circ Physiol. 2010 Jul;299(1):H1-9. doi: 10.1152/ajpheart.00335.2010. Epub 2010 May 14. Am J Physiol Heart Circ Physiol. 2010. PMID: 20472764 Review. - The Role of Lysyl Oxidase Enzymes in Cardiac Function and Remodeling.
Rodríguez C, Martínez-González J. Rodríguez C, et al. Cells. 2019 Nov 21;8(12):1483. doi: 10.3390/cells8121483. Cells. 2019. PMID: 31766500 Free PMC article. Review.
Cited by
- Megakaryocyte pathology and bone marrow fibrosis: the lysyl oxidase connection.
Papadantonakis N, Matsuura S, Ravid K. Papadantonakis N, et al. Blood. 2012 Aug 30;120(9):1774-81. doi: 10.1182/blood-2012-02-402594. Epub 2012 Jul 5. Blood. 2012. PMID: 22767499 Free PMC article. Review. - Impaired Gastric Hormone Regulation of Osteoblasts and Lysyl Oxidase Drives Bone Disease in Diabetes Mellitus.
Daley EJ, Pajevic PD, Roy S, Trackman PC. Daley EJ, et al. JBMR Plus. 2019 Aug 7;3(10):e10212. doi: 10.1002/jbm4.10212. eCollection 2019 Oct. JBMR Plus. 2019. PMID: 31687648 Free PMC article. - Common polymorphisms in human lysyl oxidase genes are not associated with the adolescent idiopathic scoliosis phenotype.
McGregor TL, Gurnett CA, Dobbs MB, Wise CA, Morcuende JA, Morgan TM, Menon R, Muglia LJ. McGregor TL, et al. BMC Med Genet. 2011 Jul 8;12:92. doi: 10.1186/1471-2350-12-92. BMC Med Genet. 2011. PMID: 21740577 Free PMC article. - The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase.
Cox TR, Rumney RMH, Schoof EM, Perryman L, Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, Huggins ID, Lang G, Linding R, Gartland A, Erler JT. Cox TR, et al. Nature. 2015 Jun 4;522(7554):106-110. doi: 10.1038/nature14492. Epub 2015 May 27. Nature. 2015. PMID: 26017313 Free PMC article. Retracted. - Genes regulated in metastatic osteosarcoma: evaluation by microarray analysis in four human and two mouse cell line systems.
Muff R, Ram Kumar RM, Botter SM, Born W, Fuchs B. Muff R, et al. Sarcoma. 2012;2012:937506. doi: 10.1155/2012/937506. Epub 2012 Nov 13. Sarcoma. 2012. PMID: 23213280 Free PMC article.
References
- Boskey AL. Matrix protein and mineralization: An overview. Conn Tissue Res. 1996;35:375–363. - PubMed
- Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem. 2004;14:2115–2123.
- Kagan H, Trackman P. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–210. - PubMed
- Prockop D, Kivirikko K. Collagens: molecular biology, diseases, and potentials for therapy. Ann Rev Biochem. 1995;64:403–434. - PubMed
- Yamauchi M. Collagen: The major matrix molecule in mineralized tissues. In: JJB A, Garner S, editors. Calcium and phosphorus in health and disease. CRC Press; New York: 1996. pp. 127–141.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases