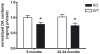Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration - PubMed (original) (raw)
doi: 10.1371/journal.pone.0005777.
Filomena Ricciardi, Alexander Kurz, Mekhman Azizov, Hans-Hermann Hoepken, Dorothea Becker, Wolfgang Voos, Kristina Leuner, Walter E Müller, Alexei P Kudin, Wolfram S Kunz, Annabelle Zimmermann, Jochen Roeper, Dirk Wenzel, Marina Jendrach, Moisés García-Arencíbia, Javier Fernández-Ruiz, Leslie Huber, Hermann Rohrer, Miguel Barrera, Andreas S Reichert, Udo Rüb, Amy Chen, Robert L Nussbaum, Georg Auburger
Affiliations
- PMID: 19492057
- PMCID: PMC2686165
- DOI: 10.1371/journal.pone.0005777
Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration
Suzana Gispert et al. PLoS One. 2009.
Abstract
Background: Parkinson's disease (PD) is an adult-onset movement disorder of largely unknown etiology. We have previously shown that loss-of-function mutations of the mitochondrial protein kinase PINK1 (PTEN induced putative kinase 1) cause the recessive PARK6 variant of PD.
Methodology/principal findings: Now we generated a PINK1 deficient mouse and observed several novel phenotypes: A progressive reduction of weight and of locomotor activity selectively for spontaneous movements occurred at old age. As in PD, abnormal dopamine levels in the aged nigrostriatal projection accompanied the reduced movements. Possibly in line with the PARK6 syndrome but in contrast to sporadic PD, a reduced lifespan, dysfunction of brainstem and sympathetic nerves, visible aggregates of alpha-synuclein within Lewy bodies or nigrostriatal neurodegeneration were not present in aged PINK1-deficient mice. However, we demonstrate PINK1 mutant mice to exhibit a progressive reduction in mitochondrial preprotein import correlating with defects of core mitochondrial functions like ATP-generation and respiration. In contrast to the strong effect of PINK1 on mitochondrial dynamics in Drosophila melanogaster and in spite of reduced expression of fission factor Mtp18, we show reduced fission and increased aggregation of mitochondria only under stress in PINK1-deficient mouse neurons.
Conclusion: Thus, aging Pink1(-/-) mice show increasing mitochondrial dysfunction resulting in impaired neural activity similar to PD, in absence of overt neuronal death.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
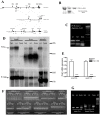
Figure 1. Generation and characterization of the Pink1 −/− mouse.
(A) Schematic drawing of the targeting strategy. (B) Demonstration of the allele representing the homologous recombination event at the Pink1 locus by Southern blotting of the outside probe in the embryonal stem cell line, (C) Presence of mutation in homozygous (hom) and heterozygous (het) mouse tail DNA represented by a double band (134+154 basepairs) instead of the wildtype (wt) single band (288 bp) after exon 4 amplification and AvaII restriction. (D) Absence of Pink1 transcript expression in three tissues of homozygous mutant versus heterozygous and wildtype mice by Northern blotting, using β-actin as control for equal loading and the 18S and 28S ribosomal bands as references for size. One additional cross-reacting band showed intensities not correlating with the mutant genotypes. No residual Pink1 transcripts of different sizes were detectable, indicating instability and degradation of the mutant mRNA. (E) 97% reduction of Pink1 mRNA in homozygous KO mice (n = 5) in independent TaqMan systems detecting sequences upstream and downstream from the Neo insertion site. (F) Saturation RT-PCR amplification of Pink1 mRNA sequences containing each splice site, studying brain and liver mRNA from wildtype, heterozygous (ht) and homozygous (hm) mice, demonstrating abnormalities in homozygous tissues with additional large bands for the exon 4–5 splicing boundary and smears of abnormally large size for the exon 5–6 boundary. (G) Saturation RT-PCR with primers from Pink1 intron 5 and the Neo selection marker demonstrating the pathological presence of intronic and Neo sequences in Pink1 mRNA from mutant (ht and hm) mouse samples.
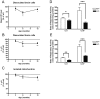
Figure 2. Progressive phenotype upon visual inspection of Pink1 −/− mice.
Knock-out (KO) versus wildtype (WT) mice showed (A) a consistent and progressive reduction of body weight from middle age and (B–F) a reduction of spontaneous movement, significant for the open-field parameters (B) total distance, (C) horizontal activity, (D) movement time, (E) stereotypy counts and (F) center distance at advanced age.
Figure 3. Aged Pink1 −/− brain contains decreased dopamine amount.
HPLC analysis of Pink1 −/− striatal homogenates detected a significant reduction of dopamine (DA) at 9 and 22–24 months of age (n = 8 for each group).
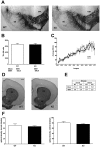
Figure 4. Aged Pink1 −/− brain does not display the typical signs of Parkinsonian neurodegeneration.
(A–C) Stereological counts of dopaminergic neurons in midbrain tissue: (A) Tyrosine hydroxylase (TH) immunostaining of wildtype (WT) and Pink1 −/− (KO) substantia nigra (SN) and ventral tegmental area (VTA) (scale bar, 200 µm). (B) Nonbiased stereological quantification of TH-immunopositive cells from WT (n = 5) and KO (n = 6) SN. The mean neuron count differed only by 113 cells. (C) Average number of unbiased sampled TH-immunopositive SN neurons in 30 serial midbrain sections, covering the caudo-rostral axis (bregma −3.8 to −3.2) in WT (n = 4) and KO (n = 6) mice. Note that there are no significant differences between the two graphs. (D–F) Optical density of dopaminergic nerve terminals in dorsal and ventral striatum: (D) TH immunostaining of striatal sections from WT and KO mice (scale bar, 500 µm). (E, F) Optical density quantification of TH immunostaining density of dorsal (DS) and ventral striatum (VS) from WT (n = 5) and KO (n = 6) mice. There were no significant differences in dorsal (mean WT 71.0, SEM 5.6; KO 66.9, SEM 2.6) and ventral striatum (WT 80.6, SEM 5.4; KO 75.7, SEM 2.5).
Figure 5. Progressive deficit in mitochondrial preprotein import.
The reduction of mitochondrial import of (OTC) precursor protein in Pink1 −/− mouse liver mitochondria (n = 2–4 animals for each group and age) was increasingly impaired with advanced age (A–C). Import of the matrix and inner membrane markers human malate dehydrogenase (MDH) (D) and cytochrome c1 (E) was reduced, while import of the inner membrane phosphate carrier protein (PiC) as marker of an alternative translocation pathway (F) was not affected in old animals.
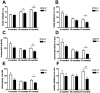
Figure 6. Progressive pathology of mitochondrial bioenergetic function in Pink1 −/− brain.
Reduced basal ATP level in Pink1 −/− dissociated cells (A), reduction of basal mitochondrial membrane potential Δψm in (B) dissociated cells and in (C) isolated mitochondria from Pink1 −/− versus wildtype brain, as well as reduction of respiratory activities for complexes I+III+IV (Cx I) and IV (Cx IV) (D,E) substantiate a mitochondrial dysfunction at old age.
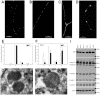
Figure 7. Early stress-induced deficit of mitochondrial fission in Pink1 −/− neurons.
Postnatal primary cortical neuron cultures stained with Mitotracker were analyzed by quantification of mitochondrial morphotypes (A = fragmented, B = tubular, C = long tubular/network, D = aggregated) under unstressed conditions (E) or after proteasomal stress with MG132 (F). Without stress, there were no differences in the mitochondrial morphology between wildtype and Pink1 −/− neuronal cells. Under stress, fewer fragmented mitochondria and significantly more aggregated mitochondria were detected in Pink1 −/− neurons, indicating a loss of stress-induced fragmentation in Pink1 −/−. Electron microscopy failed to demonstrate a loss of cristae or other conspicuous structural pathology, as seen in two representative sections from of Pink1 −/− brain at age 18 months (G, H). Immunoblots of marker proteins residing in inner (complex I–V) or outer mitochondrial membranes (porin) from 3 different mice of age 21 months did not show a proliferation of mitochondrial mass or an altered inner versus outer membrane ratio (I).
Similar articles
- Long-term oral kinetin does not protect against α-synuclein-induced neurodegeneration in rodent models of Parkinson's disease.
Orr AL, Rutaganira FU, de Roulet D, Huang EJ, Hertz NT, Shokat KM, Nakamura K. Orr AL, et al. Neurochem Int. 2017 Oct;109:106-116. doi: 10.1016/j.neuint.2017.04.006. Epub 2017 Apr 20. Neurochem Int. 2017. PMID: 28434973 Free PMC article. - Ginseng protein protects against mitochondrial dysfunction and neurodegeneration by inducing mitochondrial unfolded protein response in Drosophila melanogaster PINK1 model of Parkinson's disease.
Liu M, Yu S, Wang J, Qiao J, Liu Y, Wang S, Zhao Y. Liu M, et al. J Ethnopharmacol. 2020 Jan 30;247:112213. doi: 10.1016/j.jep.2019.112213. Epub 2019 Sep 25. J Ethnopharmacol. 2020. PMID: 31562951 - PINK1 and Parkin complementarily protect dopaminergic neurons in vertebrates.
Matsui H, Gavinio R, Asano T, Uemura N, Ito H, Taniguchi Y, Kobayashi Y, Maki T, Shen J, Takeda S, Uemura K, Yamakado H, Takahashi R. Matsui H, et al. Hum Mol Genet. 2013 Jun 15;22(12):2423-34. doi: 10.1093/hmg/ddt095. Epub 2013 Feb 27. Hum Mol Genet. 2013. PMID: 23449626 Free PMC article. - Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.
Perfeito R, Cunha-Oliveira T, Rego AC. Perfeito R, et al. Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review. - Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease.
Büeler H. Büeler H. Exp Neurol. 2009 Aug;218(2):235-46. doi: 10.1016/j.expneurol.2009.03.006. Epub 2009 Mar 18. Exp Neurol. 2009. PMID: 19303005 Review.
Cited by
- Mitochondrial dynamics and autophagy aid in removal of persistent mitochondrial DNA damage in Caenorhabditis elegans.
Bess AS, Crocker TL, Ryde IT, Meyer JN. Bess AS, et al. Nucleic Acids Res. 2012 Sep;40(16):7916-31. doi: 10.1093/nar/gks532. Epub 2012 Jun 20. Nucleic Acids Res. 2012. PMID: 22718972 Free PMC article. - Parkinson's disease: animal models and dopaminergic cell vulnerability.
Blesa J, Przedborski S. Blesa J, et al. Front Neuroanat. 2014 Dec 15;8:155. doi: 10.3389/fnana.2014.00155. eCollection 2014. Front Neuroanat. 2014. PMID: 25565980 Free PMC article. Review. - Parkin deficiency exacerbates fasting-induced skeletal muscle wasting in mice.
Peker N, Sharma M, Kambadur R. Peker N, et al. NPJ Parkinsons Dis. 2022 Nov 17;8(1):159. doi: 10.1038/s41531-022-00419-3. NPJ Parkinsons Dis. 2022. PMID: 36396647 Free PMC article. - Non-Reproducibility of Oral Rotenone as a Model for Parkinson's Disease in Mice.
Niederberger E, Wilken-Schmitz A, Manderscheid C, Schreiber Y, Gurke R, Tegeder I. Niederberger E, et al. Int J Mol Sci. 2022 Oct 21;23(20):12658. doi: 10.3390/ijms232012658. Int J Mol Sci. 2022. PMID: 36293513 Free PMC article. - Pathway-specific dopaminergic deficits in a mouse model of Angelman syndrome.
Riday TT, Dankoski EC, Krouse MC, Fish EW, Walsh PL, Han JE, Hodge CW, Wightman RM, Philpot BD, Malanga CJ. Riday TT, et al. J Clin Invest. 2012 Dec;122(12):4544-54. doi: 10.1172/JCI61888. Epub 2012 Nov 12. J Clin Invest. 2012. PMID: 23143301 Free PMC article.
References
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. - PubMed
- Bentivoglio AR, Cortelli P, Valente EM, Ialongo T, Ferraris A, et al. Phenotypic characterisation of autosomal recessive PARK6-linked parkinsonism in three unrelated Italian families. Mov Disord. 2001;16:999–1006. - PubMed
- Nakajima A, Kataoka K, Hong M, Sakaguchi M, Huh NH. BRPK, a novel protein kinase showing increased expression in mouse cancer cell lines with higher metastatic potential. Cancer Lett. 2003;201:195–201. - PubMed
- Hoepken HH, Gispert S, Morales B, Wingerter O, Del Turco D, et al. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis. 2007;25:401–411. - PubMed
- Hoepken HH, Gispert S, Azizov M, Klinkenberg M, Ricciardi F, et al. Parkinson patient fibroblasts show increased alpha-synuclein expression. Experimental neurology. 2008;212:307–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials