Enhanced cerebrovascular expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 via the MEK/ERK pathway during cerebral ischemia in the rat - PubMed (original) (raw)
Enhanced cerebrovascular expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 via the MEK/ERK pathway during cerebral ischemia in the rat
Aida Maddahi et al. BMC Neurosci. 2009.
Abstract
Background: Cerebral ischemia is usually characterized by a reduction in local blood flow and metabolism and by disruption of the blood-brain barrier in the infarct region. The formation of oedema and opening of the blood-brain barrier in stroke is associated with enhanced expression of metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1).
Results: Here, we found an infarct volume of 24.8 +/- 2% and a reduced neurological function after two hours of middle cerebral artery occlusion (MCAO), followed by 48 hours of recirculation in rat. Immunocytochemistry and confocal microscopy revealed enhanced expression of MMP-9, TIMP-1, and phosphorylated ERK1/2 in the smooth muscle cells of the ischemic MCA and associated intracerebral microvessels. The specific MEK1/2 inhibitor U0126, given intraperitoneal zero or 6 hours after the ischemic event, reduced the infarct volume significantly (11.8 +/- 2% and 14.6 +/- 3%, respectively; P < 0.05), improved neurological function, normalized expression of phosphorylated ERK1/2, and reduced expression of MMP-9 and TIMP-1 in the vessel walls. Administration of U0126 12 hours after MCAO did not alter the expression of MMP-9. Immunocytochemistry showed no overlap in expression between MMP-9/TIMP-1 and the astrocyte/glial cell marker GFAP in the vessel walls.
Conclusion: These data are the first to show that the elevated vascular expression of MMP-9 and TIMP-1, associated with breakdown of the blood-brain barrier following focal ischemia, are transcriptionally regulated via the MEK/ERK pathway.
Figures
Figure 1
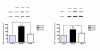
(A) Following two hours of middle cerebral artery occlusion (MCAO), the area of ischemia in animals treated with U0126 at zero or 6 hours after the start of reperfusion (b and c, respectively) are smaller than the areas area of ischemia in vehicle-treated animals (a; coronal sections stained with 2, 3, 5-triphenyltetrazolium chloride). Treatment with U0126 starting at 12 hours after MCAO (d) did not decrease the area of ischemia. (B) The infarct size (% of total brain volume) was significantly decreased in animals treated with U0126 starting at 0 hours (11.8 ± 2%**) and 6 hours (14.6 ± 3%*) after MCAO as compared to the control group (24.8 ± 2%) and animals treated with U0126 12 hours after MCAO (20.3 ± 1%). (C) Neurological assessment scores for U0126-treated rats (0, 6, and 12 hours after MCAO) and vehicle-treated rats (control). Data are expressed as mean ± SEM; n = 6–7. *P < 0.05, ** P < 0.01.
Figure 2
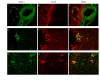
(A) Confocal microscopy images of the ischemic middle cerebral artery (MCA), cerebral micro-vessels (Mic.V), and surrounding brain tissue (Brain) immunofluorescently labeled for MMP-9 (a-f) or TIMP-1 (g-l). Images represent the vehicle control group (a, g), MCAO plus vehicle group (b, h), MCAO plus U0126 starting at 0 hours (c, i), 6 hours (d, j), or 12 hours (e, k) groups, and the negative control group (f, l). There was a significant increase in the MMP-9 protein level in the smooth muscle cell layer of ischemic vessels (MCA and Mic.V) as compared with vessels from the vehicle control group. TIMP-1 expression was upregulated in smooth muscle cells and in the proximity of the adventitia layer of ischemic vessels as compared to control vessels. Treatment with U0126, starting at zero and 6 hours, but not at 12 hours, after occlusion prevented the increase in MMP-9 and TIMP-1 protein expression. There was a slight increase in MMP-9 protein expression in ischemic brain tissue and in astrocytes around the vessels as compared to control and U0126-treated brain tissue. For TIMP-1, was no difference in protein expression in control brain tissue, in ischemic brain tissue, or tissue from animals treated with U0126. Scale bar, 50 μm. (B, C) Bar graphs showing the fluorescence intensity for MMP-9 and TIMP-1 in the MCA and micro-vessels. There was a significant increase in MMP-9 and TIMP-1 protein expression in MCAO animals as compared to control animals; this increase was prevented with U0126 treatment starting at zero and 6 hours, but not 12 hours, post MCA. Data are presented as the mean percentage relative to control ± SEM.; n = 5. *P < 0.05, **P < 0.01.
Figure 3
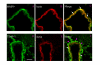
Double immunofluorescence staining for MMP-9 or TIMP-1 and actin in smooth muscle cells of the middle cerebral artery after MCAO. (A-C) Photographs demonstrating the localization of MMP-9, actin immunostaining, and their co-localization in smooth muscle cells (yellow fluorescence in the merged picture). (D-F) TIMP-1 immunostaining, actin immunostaining, and their co-localization in the smooth muscle cells (white arrows). Scale bar, 50 μm.
Figure 4
Immunoblots showing MMP-9 and TIMP-1 protein expression levels in the middle cerebral artery 48 hours after MCAO using β-actin as a loading control. Treatment with U0126 at 0 hours post occlusion decreased the MCAO-induced enhanced expression of MMP-9 and TIMP-1 receptor proteins. Data from four experiments (each performed on vessels from 3 rats) are expressed as mean ± SEM, n = 4. *P < 0.05, **P < 0.01.
Figure 5
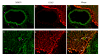
Double immunofluorescence staining for MMP-9 and GFAP demonstrate their localization in the middle cerebral artery (MCA), microvessels, and in surrounding brain tissue. MMP-9 expression in the smooth muscle cells of the MCA, micro-vessels, and brain tissue after MCAO is shown in (A) and (D) (pink arrows). GFAP expression was observed in astrocytes around the MCA, microvessels, and in the brain tissue (B and E). The merged images show the co-localization of MMP-9 and GFAP (C and F). There was no co-localization between MMP-9 and GFAP in the MCA (C), but there was some co-localization between MMP-9 and GFAP in the brain tissue and modest co-localization in the astrocytic end-feet surrounding the microvessels (white arrows).
Figure 6
Double immunofluorescence staining for TIMP-1 and GFAP show their localization in the MCA, microvessels, and brain tissue. TIMP-1 expression was seen in the cell walls of the MCA and microvessels and in nerve cells and astrocytes in the brain tissue (pink arrows; A, D, and G). GFAP expression was observed in the astrocyte cells around the MCA and microvessels and in the brain tissue (B, E, and H). Illustration of the co-localization of TIMP-1 and GFAP expression (C, F, and I). TIMP-1 immunoreactivity co-localized with GFAP immunoreactivity in the nerve cells and some areas of astrocytes in the brain tissue (C) and at the outer layer of microvessels; these areas were surrounded by astrocytic end-feet (white arrows; F and I).
Similar articles
- Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway.
Maddahi A, Edvinsson L. Maddahi A, et al. J Neuroinflammation. 2010 Feb 26;7:14. doi: 10.1186/1742-2094-7-14. J Neuroinflammation. 2010. PMID: 20187933 Free PMC article. - Enhanced expressions of microvascular smooth muscle receptors after focal cerebral ischemia occur via the MAPK MEK/ERK pathway.
Maddahi A, Edvinsson L. Maddahi A, et al. BMC Neurosci. 2008 Sep 15;9:85. doi: 10.1186/1471-2202-9-85. BMC Neurosci. 2008. PMID: 18793415 Free PMC article. - Selective knockout of astrocytic Na+ /H+ exchanger isoform 1 reduces astrogliosis, BBB damage, infarction, and improves neurological function after ischemic stroke.
Begum G, Song S, Wang S, Zhao H, Bhuiyan MIH, Li E, Nepomuceno R, Ye Q, Sun M, Calderon MJ, Stolz DB, St Croix C, Watkins SC, Chen Y, He P, Shull GE, Sun D. Begum G, et al. Glia. 2018 Jan;66(1):126-144. doi: 10.1002/glia.23232. Epub 2017 Sep 19. Glia. 2018. PMID: 28925083 Free PMC article. - The neurovascular unit, matrix proteases, and innate inflammation.
del Zoppo GJ. del Zoppo GJ. Ann N Y Acad Sci. 2010 Oct;1207:46-9. doi: 10.1111/j.1749-6632.2010.05760.x. Ann N Y Acad Sci. 2010. PMID: 20955425 Free PMC article. Review.
Cited by
- Omega-3 polyunsaturated fatty acids mitigate blood-brain barrier disruption after hypoxic-ischemic brain injury.
Zhang W, Zhang H, Mu H, Zhu W, Jiang X, Hu X, Shi Y, Leak RK, Dong Q, Chen J, Gao Y. Zhang W, et al. Neurobiol Dis. 2016 Jul;91:37-46. doi: 10.1016/j.nbd.2016.02.020. Epub 2016 Feb 24. Neurobiol Dis. 2016. PMID: 26921472 Free PMC article. - Advancement in research on the role of the transient receptor potential vanilloid channel in cerebral ischemic injury (Review).
Xie Q, Ma R, Li H, Wang J, Guo X, Chen H. Xie Q, et al. Exp Ther Med. 2021 Aug;22(2):881. doi: 10.3892/etm.2021.10313. Epub 2021 Jun 15. Exp Ther Med. 2021. PMID: 34194559 Free PMC article. Review. - Role of rho kinase in microvascular damage following cerebral ischemia reperfusion in rats.
Liu K, Li Z, Wu T, Ding S. Liu K, et al. Int J Mol Sci. 2011 Feb 18;12(2):1222-31. doi: 10.3390/ijms12021222. Int J Mol Sci. 2011. PMID: 21541054 Free PMC article. - Miconazole protects blood vessels from MMP9-dependent rupture and hemorrhage.
Yang R, Zhang Y, Huang D, Luo X, Zhang L, Zhu X, Zhang X, Liu Z, Han JY, Xiong JW. Yang R, et al. Dis Model Mech. 2017 Mar 1;10(3):337-348. doi: 10.1242/dmm.027268. Epub 2017 Feb 2. Dis Model Mech. 2017. PMID: 28153846 Free PMC article.
References
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. - PubMed
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous