Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction - PubMed (original) (raw)
Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction
Luke H Stockwin et al. Int J Cancer. 2009.
Erratum in
- Int J Cancer. 2010 Dec;127(11):E1. Bumke, Maja A [added]
Abstract
Analogs of the malaria therapeutic, artemisinin, possess in vitro and in vivo anticancer activity. In this study, two dimeric artemisinins (NSC724910 and 735847) were studied to determine their mechanism of action. Dimers were >1,000 fold more active than monomer and treatment was associated with increased reactive oxygen species (ROS) and apoptosis induction. Dimer activity was inhibited by the antioxidant L-NAC, the iron chelator desferroxamine and exogenous hemin. Similarly, induction of heme oxygenase (HMOX) with CoPPIX inhibited activity, whereas inhibition of HMOX with SnPPIX enhanced it. These results emphasize the importance of iron, heme and ROS in activity. Microarray analysis of dimer treated cells identified DNA damage, iron/heme and cysteine/methionine metabolism, antioxidant response, and endoplasmic reticulum (ER) stress as affected pathways. Detection of an ER-stress response was relevant because in malaria, artemisinin inhibits pfATP6, the plasmodium orthologue of mammalian sarcoplasmic/endoplasmic reticulum Ca(2+)-ATPases (SERCA). A comparative study of NSC735847 with thapsigargin, a specific SERCA inhibitor and ER-stress inducer showed similar behavior in terms of transcriptomic changes, induction of endogenous SERCA and ER calcium mobilization. However, thapsigargin had little effect on ROS production, modulated different ER-stress proteins and had greater potency against purified SERCA1. Furthermore, an inactive derivative of NSC735847 that lacked the endoperoxide had identical inhibitory activity against purified SERCA1, suggesting that direct inhibition of SERCA has little inference on overall cytotoxicity. In summary, these data implicate indirect ER-stress induction as a central mechanism of artemisinin dimer activity.
2009 UICC
Figures
Figure 1
Artemisinin structure and activity. A) Structures of artemisinin monomer NSC 369397 (1), artemisinin dimers NSC 724910 (2), NSC 735847 (3), and deoxy-derivative of NSC 735847 (NSC 735847DX) (4). B) Activity of artemisinin monomer and dimers. PC-3 cells were incubated with NSC 369397 (A), NSC 724910 (B) or NSC 735847 (C) for 48 h before viability was determined. C) Activity of monomer and dimers in a panel of tumor cell lines following 48 h of exposure. IC50 was defined as the concentration of drug required to inhibit [14C]-leucine incorporation by 50% relative to control-treated cells. D) The importance of the endoperoxide bridge on dimer activity. HL-60 cells (triangles) or PC-3 cells (circles) were incubated for 24 h with either NSC 735847 or NSC 735847DX before protein synthesis was determined. HL-60 cells, NSC 735847 (D), NSC 735847DX (A); PC-3 cells, NSC 735847 (C), NSC 735847DX (B). [14C]-leucine cell viability assays were performed at least twice with triplicate determinations for each point and the data pooled.
Figure 2
Artemisinin dimers increase intracellular peroxides, reduce surface transferrin receptor (CD71) and induce apoptosis without phase-specific cell cycle arrest. A) Propidium iodide (PI) cell cycle analysis of HL-60 and PC-3 cells treated with drug for 48 h was performed as described in Materials and Methods. B) For drug-treated HL-60 cells; liberation of intracellular peroxides was determined using the fluorescent substrate CM-H2DCFDA, levels of apoptosis were analyzed by staining with annexin V/propidium iodide (AnV/PI) and changes in transferrin receptor I (αTFR, CD71) expression were monitored using a fluorochrome (FITC) conjugated antibody. For generation of intracellular peroxides, cells were incubated in drug and CM-H2DCFDA for 2 h prior to analysis, whereas for apoptosis and CD71 determination cells were incubated for 24 h in compound prior to analysis. C) Modulation of heme oxygenase (HMOX1) affects NSC735847 activity. HL-60 and PC-3 cells, pre-incubated for 3 h with the HMOX1 inducer, CoPPIX, or the HMOX1 inhibitor, SnPPIX, were exposed to NSC 735847 and changes in cell viability determined using [14C]-leucine incorporation. Left panel, HL-60 cells; Right panel, PC-3 cells. (1) NSC 735847 alone, (2) NSC 735847 + 30 μM CoPPIX, (3) NSC 735847 + 30 μM SnPPIX. [14C]-leucine cell viability assays were performed at least twice with triplicate determinations for each point and the data pooled. All other assays were replicated two to three times.
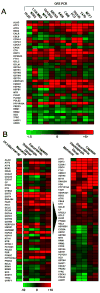
Figure 3
Real-time PCR analysis of selected transcripts identifies a conserved response to NSC735847 among different cell lines and illustrates that the response to the dimer and the SERCA inhibitor, thapsigargin, are almost identical. A) Total RNA was isolated from a panel of twelve cell lines treated with 2 μM NSC735847 for 24 h. Samples were reverse transcribed and analyzed by SYBR green real-time PCR for expression changes in selected transcripts previously identified by microarray analysis as modulated by treatment with NSC735847. Results are represented as a heat map with limits of +5 fold change (red), −5 fold change (green). B) Total RNA derived from PC-3 cells treated for 24 h with 2 μM artemisinin, 2 μM NSC735847, 0.2 μM thapsigargin, 2 μM doxorubicin or 2 μM cisplatin was isolated and reverse transcribed to cDNA. The left column shows results from the microarray analysis of dimer-treated PC-3 cells. The results were plotted (left panel) in terms of a heat map with limits of +10 change (red) and −10 fold change (green). Gene expression data was subjected to hierarchical cluster analysis (right panel) showing that the highest degree of homology between treatments was for NSC735847 and thapsigargin. Data shown is representative of that obtained over two replicate experiments using different cDNA samples where amplifications were measured in triplicate.
Figure 4
NSC735847 and thapsigargin have mechanistic differences. A) Lysates prepared from PC-3 cells treated with the indicated concentrations of NSC735847 or thapsigargin were probed for ER stress and UPR proteins. Left panel, western blot; right panel, densitometric analysis of the blots using Kodak Molecular Imaging software. Activating transcription factor 6 (ATF6), calnexin (CANX), caspase 12 (CASP12), heat shock 70 kDa protein 5 (HSPA5/GRP78) and homocysteine-responsive endoplasmic reticulum-resident ubiquitin-like domain member 1 (HERPUD1). Control, β-actin (ACTB). B) Generation of intracellular peroxides in HL-60 and PC-3 cells treated for 2 h with NSC 735847 (2 μM) or thapsigargin (1 μM) as determined using the fluorescent substrate CM-H2DCFDA. Solid histograms show drug treated cells, open histograms are control samples. C) The effect of the antioxidant L-NAC on the activity of NSC735847 and thapsigargin as determined by [14C]-leucine viability assays. Cells were pretreated with 11 mM L-NAC for 1 h before the addition of NSC735847 or thapsigargin and the incorporation of [14C]-leucine determined after 24 h. Top panel, PC-3; Bottom panel, HL-60 cells. Thapsigargin (1), thapsigargin + L-NAC (2), NSC735847 (3), NSC735847 + L-NAC (4). -[14C]-leucine cell viability assays were performed at least twice with triplicate determinations for each point and the data pooled. All other assays were replicated two to three times.
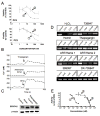
Figure 5
Exploring the role of SERCA Ca2+-ATPases in NSC735847 activity. A) Calcium chelation attenuates cytotoxicity of thapsigargin and NSC735847 as determined by [14C]-leucine viability assays. PC-3 cells were either preincubated with 100 μM BAPTA-AM for 1 h before addition of NSC735847 and the incubation continued for 23 h, or the cells were preincubated with 50 μM BAPTA-AM and 50 μM EGTA-AM for 1 h followed by the addition of thapsigargin for 9 h. Cells were then washed and the incubation continued for 14 h. Top panel, NSC735847 (1), NSC735847 + 100 μM BAPTA-AM (2); bottom panel, thapsigargin (1), thapsigargin + 50 μM BAPTA-AM and 50 μM EGTA-AM (2). B) Comparison of the effect of NSC735847 with that of the SERCA inhibitors, thapsigargin and BHQ, on the intracellular calcium concentration [Ca2+]i in PC-3 cells. Thapsigargin, NSC735847 and BHQ increase [Ca2+]i of PC-3 cells, loaded with Fluo-3. The tracings show the change in [Ca2+]i from the fluorescence of Fluo-3 loaded PC-3 cells in the presence of extracellular Ca2+ (1 mM). Fluo-3/AM fluorescence was recorded every 20 sec in duplicate and the readings averaged. C) Effects of treatment on expression of SERCA2. PC-3 cells were incubated for 24 h with NSC735847 (16 μM) or thapsigargin (100 nM) and total cell lysates probed by western blotting for expression of SERCA2 and β-actin. D) Dimer treatment induces oxidative modification of SERCA. Aliquots (50 μg) of SERCA were treated with increasing concentration of artemisinin, NSC735847, NSC735847DX or thapsigargin, and incubated over night at 37°C. Protein samples were labeled with ThioGlo1, a fluorescent reduced cysteine reactive molecule. After 2 h, labeled protein samples were resolved by SDS-PAGE and fluorescence intensities quantified by UV transillumination and densitometry. E) NSC735847 and NSC735847DX have superimposable SERCA inhibitory activity. Ca2+-dependent ATPase activity was measured with 15.5 μg/mL rat skeletal muscle SR at varying concentrations of thapsigargin (1), NSC735847 (2), or NSC735847DX (3) by a coupled enzyme assay. -[14C]-leucine cell viability assays were performed at least twice with triplicate determinations for each point and the data pooled. All other assays were replicated two to three times.
Similar articles
- Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.
Haynes RK, Cheu KW, N'Da D, Coghi P, Monti D. Haynes RK, et al. Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review. - Enhanced endoplasmic reticulum SERCA activity by overexpression of hepatic stimulator substance gene prevents hepatic cells from ER stress-induced apoptosis.
Zhang J, Li Y, Jiang S, Yu H, An W. Zhang J, et al. Am J Physiol Cell Physiol. 2014 Feb 1;306(3):C279-90. doi: 10.1152/ajpcell.00117.2013. Epub 2013 Nov 27. Am J Physiol Cell Physiol. 2014. PMID: 24284796 - N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury.
Sun Y, Pu LY, Lu L, Wang XH, Zhang F, Rao JH. Sun Y, et al. World J Gastroenterol. 2014 Nov 7;20(41):15289-98. doi: 10.3748/wjg.v20.i41.15289. World J Gastroenterol. 2014. PMID: 25386077 Free PMC article. - The SERCA pump as a therapeutic target: making a "smart bomb" for prostate cancer.
Denmeade SR, Isaacs JT. Denmeade SR, et al. Cancer Biol Ther. 2005 Jan;4(1):14-22. doi: 10.4161/cbt.4.1.1505. Epub 2005 Jan 23. Cancer Biol Ther. 2005. PMID: 15662118 Review.
Cited by
- pH-responsive artemisinin derivatives and lipid nanoparticle formulations inhibit growth of breast cancer cells in vitro and induce down-regulation of HER family members.
Zhang YJ, Gallis B, Taya M, Wang S, Ho RJ, Sasaki T. Zhang YJ, et al. PLoS One. 2013;8(3):e59086. doi: 10.1371/journal.pone.0059086. Epub 2013 Mar 14. PLoS One. 2013. PMID: 23516601 Free PMC article. - Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells.
Morrissey C, Gallis B, Solazzi JW, Kim BJ, Gulati R, Vakar-Lopez F, Goodlett DR, Vessella RL, Sasaki T. Morrissey C, et al. Anticancer Drugs. 2010 Apr;21(4):423-32. doi: 10.1097/CAD.0b013e328336f57b. Anticancer Drugs. 2010. PMID: 20130467 Free PMC article. - The molecular mechanism of action of artemisinin--the debate continues.
O'Neill PM, Barton VE, Ward SA. O'Neill PM, et al. Molecules. 2010 Mar 12;15(3):1705-21. doi: 10.3390/molecules15031705. Molecules. 2010. PMID: 20336009 Free PMC article. Review. - Artemisinin analogues as potent inhibitors of in vitro hepatitis C virus replication.
Obeid S, Alen J, Nguyen VH, Pham VC, Meuleman P, Pannecouque C, Le TN, Neyts J, Dehaen W, Paeshuyse J. Obeid S, et al. PLoS One. 2013 Dec 11;8(12):e81783. doi: 10.1371/journal.pone.0081783. eCollection 2013. PLoS One. 2013. PMID: 24349127 Free PMC article. - Advances in the research on the targets of anti-malaria actions of artemisinin.
Yang J, He Y, Li Y, Zhang X, Wong YK, Shen S, Zhong T, Zhang J, Liu Q, Wang J. Yang J, et al. Pharmacol Ther. 2020 Dec;216:107697. doi: 10.1016/j.pharmthera.2020.107697. Epub 2020 Oct 6. Pharmacol Ther. 2020. PMID: 33035577 Free PMC article. Review.
References
- White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–4. - PubMed
- Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–55. - PubMed
- Li Y, Wu YL. How Chinese scientists discovered qinghaosu (artemisinin) and developed its derivatives? What are the future perspectives? Med Trop (Mars) 1998;58:9–12. - PubMed
- Efferth T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr Drug Targets. 2006;7:407–21. - PubMed
- Paik IH, Xie S, Shapiro TA, Labonte T, Narducci Sarjeant AA, Baege AC, Posner GH. Second generation, orally active, antimalarial, artemisinin-derived trioxane dimers with high stability, efficacy, and anticancer activity. J Med Chem. 2006;49:2731–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous