Identification of residues in the drug translocation pathway of the human multidrug resistance P-glycoprotein by arginine mutagenesis - PubMed (original) (raw)
Identification of residues in the drug translocation pathway of the human multidrug resistance P-glycoprotein by arginine mutagenesis
Tip W Loo et al. J Biol Chem. 2009.
Abstract
P-glycoprotein (P-gp, ATP-binding cassette B1) is a drug pump that extracts toxic drug substrates from the plasma membrane and catalyzes their ATP-dependent efflux. To map the residues in the drug translocation pathway, we performed arginine-scanning mutagenesis on all transmembrane (TM) segments (total = 237 residues) of a P-gp processing mutant (G251V) defective in folding (15% maturation efficiency) (glycosylation state used to monitor folding). The rationale was that arginines introduced into the drug-binding sites would mimic drug rescue and enhance maturation of wild-type or processing mutants of P-gp. It was found that 38 of the 89 mutants that matured had enhanced maturation. Enhancer mutations were found in 11 of the 12 TM segments with the largest number found in TMs 6 and 12 (seven in each), TMs that are critical for P-gp-drug substrate interactions. Modeling of the TM segments showed that the enhancer arginines were found on the hydrophilic face, whereas inhibitory arginines were located on a hydrophobic face that may be in contact with the lipid bilayer. It was found that many of the enhancer arginines caused large alterations in P-gp-drug interactions in ATPase assays. For example, mutants A302R (TM5), L339R (TM6), G872R (TM10), F942R (TM11), Q946R (TM11), V982R (TM12), and S993R (TM12) reduced the apparent affinity for verapamil by approximately 10-fold, whereas the F336R (TM6) and M986R (TM12) mutations caused at least a 10-fold increase in apparent affinity for rhodamine B. The results suggest that P-gp contains a large aqueous-filled drug translocation pathway with multiple drug-binding sites that can accommodate the bulky arginine side chains to promote folding of the protein.
Figures
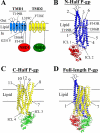
FIGURE 1.
Schematic models of P-gp. A, the 12 TMs of P-gp are shown as numbered cylinders with those in TMD1 shaded in blue and those in TMD2 shaded in yellow. The NBDs are depicted as ovals. The branched lines on the loop connecting TMs 1 and 2 represent the glycosylated sites. The location of the G251Vprocessing mutation is indicated as a square. The positions of arginines that were previously found to promote maturation of P-gp processing mutants (T199R (TM3), I306R (TM5), and F343R (TM6)) (26) are indicated as filled circles. Cysteines that act as reporter sites for binding of drug substrates (L339C (TM6) and F728C (TM7)) (22) are indicated as open circles. B–D, the predicted models (6) of N-half (B), C-half (C), and full-length (D) P-gps in the predicted closed conformations (NBDs close together) are shown. The locations of the predicted intracellular loops (ICL) and TM segments in the half-molecules (B and C) are shown.
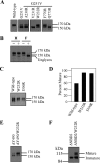
FIGURE 2.
Identification of arginine mutations that promote, inhibit, or have little effect on maturation of mutant G251V. A, 48 h after transfection, whole cell SDS extracts of HEK 293 cells expressing A52-tagged wild-type P-gp (Wild-type), mutant G251V, mutants A230R/G251V, V231R/G251V, or W232R/G251V in TM4 or mutants F770R/G251V or Q773R/G251V in TM8 were subjected to immunoblot analysis with monoclonal antibody A52. B, 48 h after transfection, whole cell SDS extracts of HEK 293 cells expressing mutant W232R/G251V were treated with (+) endoglycosidase Hf (H), peptide _N_-glycosidase F (F), or no (−) endoglycosidase. Equivalent amounts were subjected to immunoblot analysis. C, 24 h after transfection, whole cell SDS extracts of HEK 293 cells expressing A52-tagged wild-type P-gp (Wild-type), mutant W232R in TM4, or I306R in TM5 were subjected to immunoblot analysis. D–E, the amount of mature P-gp (170-kDa protein) relative to total (mature 170-kDa protein plus immature 150-kDa protein) (% mature) was quantitated (D). Forty-eight hours after transfection, whole cell SDS extracts of HEK 293 cells expressing A52-tagged mutants ΔY490 or ΔY490/W232R (E), mutants ΔNBD2 (deleted residues 1024–1280) or ΔNBD2/W232R (F) were subjected to immunoblot analysis. The positions of the mature (170 kDa), immature (150 kDa), and unglycosylated (Unglycos) P-gps are indicated.
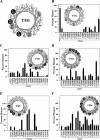
FIGURE 3.
Effect of TMD1 arginine mutations on maturation of mutant G251V. Whole cell SDS extracts of cells expressing wild-type P-gp (Wild-type), mutant G251V, or G251V mutants containing arginines at various positions in predicted TM segments 1 (A), 2 (B), 3 (C), 4 (D), 5 (E), or 6 (F) of TMD1 were subjected to immunoblot analysis, and the relative amount of mature P-gp was determined. The amount of mature P-gp (170-kDa protein) relative to total (mature 170-kDa protein plus immature 150-kDa protein) (% mature) was quantitated. Each value is the mean ± S.D. (n = 3–5). The insets show the positions of the residues in the TM segments as α-helical wheels and the effect of arginine mutations at various positions on maturation of mutant G251V. Arginine mutations that inhibit, promote, or have a neutral effect on maturation are shown as white circles, black circles, or gray circles, respectively. The helical wheel for TM1 (A) summarizes previous data (10) and is included for comparison.
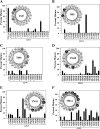
FIGURE 4.
Effect of TMD2 arginine mutations on maturation of mutant G251V. Maturation efficiency of wild-type P-gp (Wild-type), mutant G251V, or G251V mutants containing arginines at various positions in predicted TM segments 7 (‘A), 8 (B), 9 (C), 10 (D), 11 (E), or 12 (F) of TMD2 were determined as described in the legend for Fig. 3. The insets show the predicted positions of the residues in the TM segments as α-helical wheel structures and the effect of arginine mutations at various positions on maturation of mutant G251V. Arginine mutations that inhibit, promote, or have a neutral effect on maturation are shown as white circles, black circles, or gray circles, respectively.
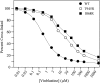
FIGURE 5.
Drug-stimulated ATPase activities of wild-type and mutant M986R P-gps. The M986R (TM12) mutation was introduced into a wild-type P-gp background. Histidine-tagged wild-type (WT) or mutant M986R (TM12) P-gps were expressed in HEK 293 cells and isolated by nickel-chelate chromatography. The isolated P-gps were mixed with lipid, and ATPase activities were measured in the presence of various concentrations of vinblastine, verapamil, colchicine, or rhodamine B. Each value is the average of triplicate experiments.
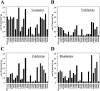
FIGURE 6.
Effect of enhancer arginines on maximum stimulation of ATPase activity by drug substrates. Arginine enhancer mutations were introduced into a wild-type P-gp background. Histidine-tagged wild-type or mutant P-gps were expressed in HEK 293 cells and isolated by nickel-chelate chromatography. The isolated P-gps were mixed with lipid, and ATPase activities were measured in the presence of various concentrations drug substrate. The maximum activities observed with verapamil (A), vinblastine (B), colchicine (C), or rhodamine B (D) are shown. Each value is the average of triplicate experiments.
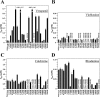
FIGURE 7.
Effect of enhancer arginines on apparent affinity of P-gp for drug substrates. Arginine enhancer mutations were introduced into a wild-type P-gp background. Histidine-tagged wild-type or mutant P-gps were expressed in HEK 293 cells and isolated by nickel-chelate chromatography. The isolated P-gps were mixed with lipid, and ATPase activities were measured in the presence of various concentrations drug substrates. The concentrations of verapamil (A), vinblastine (B), colchicine (C), or rhodamine B (D) required to stimulate ATPase activity by 50% (_S_50) are shown. Each value is the mean ± S.D. (n = 3).
FIGURE 8.
Effect of I868R and T945R mutations on inhibition of cross-linking by vinblastine. The membranes were prepared from HEK 293 cells expressing mutants L339C (TM6)/F728C (TM7), I868R (TM10)/L339C (TM6)/F728C (TM7), or T945R (TM11)/L339C (TM6)/F728C (TM7). The samples were incubated with various concentrations of vinblastine at 20 °C. The samples were cooled on ice and then treated with 0.2 m
m
M14M for 4 min on ice. The reactions were stopped by the addition of SDS sample buffer containing no thiol-reducing agent. The gel lanes were scanned and analyzed with the NIH Image program and an Apple Macintosh computer. The Percent cross-linked is the amount of cross-linked P-gp in the presence of drug substrate relative to that with no drug substrate. WT, wild type.
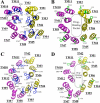
FIGURE 9.
Models showing the locations of introduced arginine residues in the TM segments of human or mouse P-gps. The cytoplasmic portions of TM segments of human (A and B) (6) or mouse (C and D) (8) P-gp are shown and viewed from the extracellular side of the protein. The TM segments of human P-gp shown are Met51–His61 (TM1), Ile131–Gly141 (TM2), Ile190–Phe200 (TM3), Pro223–Ala233 (TM4), Asn296–Ile306 (TM5), Phe336–Ile352 (TM6), Val712–Asn721 (TM7), Ser766–Phe777 (TM8), Leu833–Gly846 (TM9), Ile864–Glu875 (TM10), Phe938–Met949 (TM11), and Ala980–Val991 (TM12). The TM segments of mouse MDR1a P-gp shown are Leu51- His60 (TM1), Ile127–Gly137 (TM2), Ile186–Phe196 (TM3), Pro219–Ala229 (TM4), Asn292–Ile302 (TM5), Phe332–Ile348 (TM6), Val708–Asn717 (TM7), Ser762–Phe773 (TM8), Leu829–Gly842 (TM9), Ile860–Glu871 (TM10), Phe934–Met945 (TM11), and Ala976–Val987 (TM12). The side chains of residues at positions where arginine mutations promoted or inhibited maturation are shown in blue (A and C) or green (B and D), respectively. The positions of the equivalent residues in mouse and human P-gps are indicated.
Similar articles
- Arginines in the first transmembrane segment promote maturation of a P-glycoprotein processing mutant by hydrogen bond interactions with tyrosines in transmembrane segment 11.
Loo TW, Bartlett MC, Clarke DM. Loo TW, et al. J Biol Chem. 2008 Sep 5;283(36):24860-70. doi: 10.1074/jbc.M803351200. Epub 2008 Jul 2. J Biol Chem. 2008. PMID: 18596043 Free PMC article. - Insertion of an arginine residue into the transmembrane segments corrects protein misfolding.
Loo TW, Bartlett MC, Clarke DM. Loo TW, et al. J Biol Chem. 2006 Oct 6;281(40):29436-40. doi: 10.1074/jbc.C600209200. Epub 2006 Aug 22. J Biol Chem. 2006. PMID: 16926162 - Suppressor mutations in the transmembrane segments of P-glycoprotein promote maturation of processing mutants and disrupt a subset of drug-binding sites.
Loo TW, Bartlett MC, Clarke DM. Loo TW, et al. J Biol Chem. 2007 Nov 2;282(44):32043-52. doi: 10.1074/jbc.M706175200. Epub 2007 Sep 11. J Biol Chem. 2007. PMID: 17848563 - Structural and functional properties of human multidrug resistance protein 1 (MRP1/ABCC1).
He SM, Li R, Kanwar JR, Zhou SF. He SM, et al. Curr Med Chem. 2011;18(3):439-81. doi: 10.2174/092986711794839197. Curr Med Chem. 2011. PMID: 21143116 Review. - Determining the structure and mechanism of the human multidrug resistance P-glycoprotein using cysteine-scanning mutagenesis and thiol-modification techniques.
Loo TW, Clarke DM. Loo TW, et al. Biochim Biophys Acta. 1999 Dec 6;1461(2):315-25. doi: 10.1016/s0005-2736(99)00165-0. Biochim Biophys Acta. 1999. PMID: 10581364 Review.
Cited by
- Multiple Drug Transport Pathways through Human P-Glycoprotein.
McCormick JW, Vogel PD, Wise JG. McCormick JW, et al. Biochemistry. 2015 Jul 21;54(28):4374-90. doi: 10.1021/acs.biochem.5b00018. Epub 2015 Jul 10. Biochemistry. 2015. PMID: 26125482 Free PMC article. - Transmembrane helices 1 and 6 of the human breast cancer resistance protein (BCRP/ABCG2): identification of polar residues important for drug transport.
Ni Z, Bikadi Z, Cai X, Rosenberg MF, Mao Q. Ni Z, et al. Am J Physiol Cell Physiol. 2010 Nov;299(5):C1100-9. doi: 10.1152/ajpcell.00160.2010. Epub 2010 Aug 25. Am J Physiol Cell Physiol. 2010. PMID: 20739628 Free PMC article. - Mapping the Binding Site of the Inhibitor Tariquidar That Stabilizes the First Transmembrane Domain of P-glycoprotein.
Loo TW, Clarke DM. Loo TW, et al. J Biol Chem. 2015 Dec 4;290(49):29389-401. doi: 10.1074/jbc.M115.695171. Epub 2015 Oct 26. J Biol Chem. 2015. PMID: 26507655 Free PMC article. - Human P-glycoprotein contains a greasy ball-and-socket joint at the second transmission interface.
Loo TW, Bartlett MC, Clarke DM. Loo TW, et al. J Biol Chem. 2013 Jul 12;288(28):20326-33. doi: 10.1074/jbc.M113.484550. Epub 2013 Jun 3. J Biol Chem. 2013. PMID: 23733192 Free PMC article. - Extended-ensemble docking to probe dynamic variation of ligand binding sites during large-scale structural changes of proteins.
Kapoor K, Thangapandian S, Tajkhorshid E. Kapoor K, et al. Chem Sci. 2022 Mar 16;13(14):4150-4169. doi: 10.1039/d2sc00841f. eCollection 2022 Apr 6. Chem Sci. 2022. PMID: 35440993 Free PMC article.
References
- Ambudkar S. V., Dey S., Hrycyna C. A., Ramachandra M., Pastan I., Gottesman M. M. (1999) Annu. Rev. Pharmacol. Toxicol. 39, 361–398 - PubMed
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. (1986) Cell 47, 381–389 - PubMed
- Loo T. W., Clarke D. M. (1999) J. Biol. Chem. 274, 24759–24765 - PubMed
- Loo T. W., Clarke D. M. (1998) J. Biol. Chem. 273, 14671–14674 - PubMed
- Loo T. W., Clarke D. M. (2008) Arch. Biochem. Biophys. 476, 51–64 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous