Lysyl oxidase interacts with AGE signalling to modulate collagen synthesis in polycystic ovarian tissue - PubMed (original) (raw)
Lysyl oxidase interacts with AGE signalling to modulate collagen synthesis in polycystic ovarian tissue
Katerina K Papachroni et al. J Cell Mol Med. 2010 Oct.
Abstract
Connective tissue components--collagen types I, III and IV--surrounding the ovarian follicles undergo drastic changes during ovulation. Abnormal collagen synthesis and increased volume and density of ovarian stroma characterize the polycystic ovary syndrome (PCOS). During the ovulatory process, collagen synthesis is regulated by prolyl hydroxylase and lysyl oxidase (LOX) activity in ovarian follicles. LOX catalyzes collagen and elastin cross-linking and plays essential role in coordinating the control of ovarian extracellular matrix (ECM) during follicular development. We have recently shown accumulation of advanced glycation end products (AGEs), molecules that stimulate ECM production and abnormal collagen cross-linking, in ovarian tissue. However, the possible link between LOX and AGEs-induced signalling in collagen production and stroma formation in ovarian tissue from PCOS remains elusive. The present study investigates the hypothesis of AGE signalling pathway interaction with LOX gene activity in polycystic ovarian (PCO) tissue. We show an increased distribution and co-localization of LOX, collagen type IV and AGE molecules in the PCO tissue compared to control, as well as augmented expression of AGE signalling mediators/effectors, phospho(p)-ERK, phospho(p)-c-Jun and nuclear factor κB (NF-κB) in pathological tissue. Moreover, we demonstrate binding of AGE-induced transcription factors, NF-κB and activator protein-1 (AP-1) on LOX promoter, indicating a possible involvement of AGEs in LOX gene regulation, which may account for the documented increase in LOX mRNA and protein levels compared to control. These findings suggest that deposition of excess collagen in PCO tissue that induces cystogenesis may, in part, be due to AGE-mediated stimulation of LOX activity.
© 2009 The Authors Journal compilation © 2010 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
Fig 1
Immunodetection of collagen type IV and LOX in PCO and control samples. Immunohistochemistry was performed on sections from stromal (top panels) or follicular (bottom panel) ovarian tissue, which were stained for collagen type IV (A) and LOX (B). Increased selective staining is observed in PCO sections (magnification ×100). Pictures are representative of 20 sections. (C) Immunohistochemical detection of AGEs in polycystic ovaries [24]. Positive staining is observed in the follicular cell layers (granulosa and theca layers), luteinized cells and in endothelial cells of polycystic ovaries, with stronger staining intensity observed in granulosa cells compared to normal tissue (_P_= 0.036). AGE immunoreactivity positively correlates with LOX staining in granulosa cells (_R_= 0.285, _P_= 0.041). (D) Western immunoblotting of 40 μg of whole-cell extract from control and PCO tissue against anti-collagen type IV and anti-LOX antibodies. Collagen type IV protein expression is increased by 3-fold and LOX protein expression by approximately 4-fold in the PCO tissue relative to control.
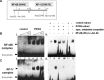
Fig 3
Induced NF-κB and AP-1 binding to LOX promoter in PCO tissue. (A) Line diagram of part of the LOX promoter. The binding sites for the NF-κB and AP-1 transcription factors are indicated, as well as the restriction enzymes are used to isolate the respective DNA fragments from E. coli transfected cells. (B, C) EMSA with the NF-κβ p65 DNA biotin-labelled probe (Bi) or the AP-1/c-Jun biotin-labelled probe (Ci) and whole-cell ovarian protein extract from human samples of healthy (control) and PCO tissue. Increasing concentrations (10, 20, 40 μg) of protein were used (lanes 1–3 for control and 4–6 for PCO tissue protein extract) and the LOX promoter – NF-κB and LOX promoter – AP-1 complex is indicated in the two panels, respectively. (Bii, Cii) Binding competition assays with 100-fold (lane 9) and 200-fold (lane 10) molar excess of specific unlabelled probe reacting with protein extract from PCO tissue. Note that in the lower panel (AP-1 probe, Cii), lane 9* contains 200-fold molar excess and lane 10* contains 100-fold molar excess of specific unlabelled probe. Lanes 11 and 12 correspond to reactions in which pre-incubation of control and PCO tissue protein extract with anti-NF-κB p65 or anti-p-c-Jun antibody was performed, before the addition of the labelled probe.
Fig 2
Immunodetection of p-ERK, p-c-Jun and NF-κB p65 in PCO and control samples. (A) Sections of ovarian tissue were immunostained for p-ERK, p-c-Jun and NF-κB p65. Increased staining is observed on the pathological tissue. Pictures are representative of 20 sections (magnification ×100). (B) Western immunoblotting of 40 μg of whole-cell extract from control and PCO tissue incubated with anti-p-ERK, anti-p-c-Jun and anti-NF-κB p65 antibodies. Protein levels of p-ERK are increased by 3-fold, of p-c-Jun 1.5-fold and of NF-κB p65 2-fold relative to control.
Fig 4
Induction of the LOX mRNA and LOX protein levels in the pathological ovarian tissue. (A) Real-time PCR was employed to achieve relative quantification of LOX mRNA in control and PCO tissue. Using the Pfaffl equation increase in LOX mRNA expression was calculated as 3-fold relative to control. Representative graph of the melting curves of the LOX and GAPDH PCR products. (B) LOX protein levels are increased by almost 4-fold in PCO tissue as revealed by Western immunoblotting.
Similar articles
- Advanced glycation end products upregulate lysyl oxidase and endothelin-1 in human aortic endothelial cells via parallel activation of ERK1/2-NF-κB and JNK-AP-1 signaling pathways.
Adamopoulos C, Piperi C, Gargalionis AN, Dalagiorgou G, Spilioti E, Korkolopoulou P, Diamanti-Kandarakis E, Papavassiliou AG. Adamopoulos C, et al. Cell Mol Life Sci. 2016 Apr;73(8):1685-98. doi: 10.1007/s00018-015-2091-z. Epub 2015 Dec 8. Cell Mol Life Sci. 2016. PMID: 26646068 Free PMC article. - Lysyl oxidase and MMP-2 expression in dehydroepiandrosterone-induced polycystic ovary in rats.
Henmi H, Endo T, Nagasawa K, Hayashi T, Chida M, Akutagawa N, Iwasaki M, Kitajima Y, Kiya T, Nishikawa A, Manase K, Kudo R. Henmi H, et al. Biol Reprod. 2001 Jan;64(1):157-62. doi: 10.1095/biolreprod64.1.157. Biol Reprod. 2001. PMID: 11133670 - Lysyl oxidase blockade ameliorates anovulation in polycystic ovary syndrome.
Zhang C, Ma J, Wang W, Sun Y, Sun K. Zhang C, et al. Hum Reprod. 2018 Nov 1;33(11):2096-2106. doi: 10.1093/humrep/dey292. Hum Reprod. 2018. PMID: 30272163 - Exploring the Interplay between Polyphenols and Lysyl Oxidase Enzymes for Maintaining Extracellular Matrix Homeostasis.
Añazco C, Riedelsberger J, Vega-Montoto L, Rojas A. Añazco C, et al. Int J Mol Sci. 2023 Jul 1;24(13):10985. doi: 10.3390/ijms241310985. Int J Mol Sci. 2023. PMID: 37446164 Free PMC article. Review. - Lysyl oxidases: from enzyme activity to extracellular matrix cross-links.
Vallet SD, Ricard-Blum S. Vallet SD, et al. Essays Biochem. 2019 Sep 13;63(3):349-364. doi: 10.1042/EBC20180050. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31488698 Review.
Cited by
- Cardiac-restricted Overexpression of TRAF3 Interacting Protein 2 (TRAF3IP2) Results in Spontaneous Development of Myocardial Hypertrophy, Fibrosis, and Dysfunction.
Yariswamy M, Yoshida T, Valente AJ, Kandikattu HK, Sakamuri SS, Siddesha JM, Sukhanov S, Saifudeen Z, Ma L, Siebenlist U, Gardner JD, Chandrasekar B. Yariswamy M, et al. J Biol Chem. 2016 Sep 9;291(37):19425-36. doi: 10.1074/jbc.M116.724138. Epub 2016 Jul 27. J Biol Chem. 2016. PMID: 27466370 Free PMC article. - Emerging concepts about prenatal genesis, aberrant metabolism and treatment paradigms in polycystic ovary syndrome.
Witchel SF, Recabarren SE, González F, Diamanti-Kandarakis E, Cheang KI, Duleba AJ, Legro RS, Homburg R, Pasquali R, Lobo RA, Zouboulis CC, Kelestimur F, Fruzzetti F, Futterweit W, Norman RJ, Abbott DH. Witchel SF, et al. Endocrine. 2012 Dec;42(3):526-34. doi: 10.1007/s12020-012-9701-4. Epub 2012 Jun 4. Endocrine. 2012. PMID: 22661293 Free PMC article. Review. - Advanced glycation end products upregulate lysyl oxidase and endothelin-1 in human aortic endothelial cells via parallel activation of ERK1/2-NF-κB and JNK-AP-1 signaling pathways.
Adamopoulos C, Piperi C, Gargalionis AN, Dalagiorgou G, Spilioti E, Korkolopoulou P, Diamanti-Kandarakis E, Papavassiliou AG. Adamopoulos C, et al. Cell Mol Life Sci. 2016 Apr;73(8):1685-98. doi: 10.1007/s00018-015-2091-z. Epub 2015 Dec 8. Cell Mol Life Sci. 2016. PMID: 26646068 Free PMC article. - Polycystic ovarian syndrome (PCOS) and recurrent spontaneous abortion (RSA) are associated with the PI3K-AKT pathway activation.
Lin W, Wang Y, Zheng L. Lin W, et al. PeerJ. 2024 Sep 6;12:e17950. doi: 10.7717/peerj.17950. eCollection 2024. PeerJ. 2024. PMID: 39253602 Free PMC article. - Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways.
Mouanness M, Merhi Z. Mouanness M, et al. Nutrients. 2022 Feb 24;14(5):966. doi: 10.3390/nu14050966. Nutrients. 2022. PMID: 35267940 Free PMC article. Review.
References
- Diamanti-Kandarakis E, Piperi C, Patsouris E, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. - PubMed
- Clement F, Monniaux D, Thalabard JC, et al. Contribution of a mathematical modelling approach to the understanding of the ovarian function. C R Biol. 2002;325:473–85. - PubMed
- Auersperg N, Maines-Bandiera SL, Dyck HG, et al. Characterization of cultured human ovarian surface epithelial cells: phenotypic plasticity and premalignant changes. Lab Invest. 1994;71:510–8. - PubMed
- Kruk PA, Auersperg N. A line of rat ovarian surface epithelium provides a continuous source of complex extracellular matrix. In Vitro Cell Dev Biol Anim. 1994;30A:217–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous