Myocardial lysyl oxidase regulation of cardiac remodeling in a murine model of diet-induced metabolic syndrome - PubMed (original) (raw)
Myocardial lysyl oxidase regulation of cardiac remodeling in a murine model of diet-induced metabolic syndrome
Sherma Zibadi et al. Am J Physiol Heart Circ Physiol. 2009 Sep.
Abstract
Metabolic syndrome (MetS) represents an increased risk of cardiovascular disease. Although its individual components adversely affect cardiac structure and function, the extent to which multiple components of MetS affect the cardiac extracellular matrix (ECM) has not been well characterized. Lysyl oxidase (LOX) is one of the cardiac ECM-modifying enzymes that catalyze the formation of collagen cross-linking. Our objective was to define the effect of diet-induced MetS on the LOX enzyme. MetS was induced in male C57BL/6 mice by administrating a high-fat, high-simple carbohydrate diet for 6 mo. Gene expression was determined by real-time PCR. The cardiac protein expression and enzymatic activity of LOX were measured. The severity of fibrosis was assessed by histology and hydroxylproline assay. Cardiac diastolic function was assessed by in vivo analysis of the pressure-volume relationship. LOX, matrix metalloproteinases, and their tissue inhibitors were analyzed, and of these three, LOX was most significantly changed in the MetS mice. Despite the blunted gene expression of LOX isoforms, MetS mice demonstrated a significant upregulation of bone morphogenetic protein-1. Correspondingly, there was an increase in the ratio of protein expression of mature to proenzyme LOX by 25.9%, enhanced LOX activity by 50.0%, and increased cardiac cross-linked collagen compared with the controls. This fibrotic response coincided with a marked increase in end-diastolic pressure, increased left ventricular stiffness, and impaired diastolic filling pattern. Our data signify that diet-induced MetS alters the remodeling enzymes, mainly LOX, thereby altering ECM structure by increasing the amount of cross-linking and inducing diastolic dysfunction.
Figures
Fig. 1.
In vivo analysis of diastolic function. Data represent diastolic functional parameters obtained from in vivo pressure-volume (P-V) loop analysis. High-fat, high-simple carbohydrate (HFHSC) diet was associated with a significant decrease in end-diastolic volume (A), an increase in end-diastolic pressure (B), an increase in the slope of end-diastolic P-V relationship (β; C), and a reduction of left ventricular (LV) filling rate (D). There was also an upward and leftward shift of LV P-V loop (E). Values are presented as means ± SE; n = 4–8 mice/group. *P <0.05 and †P <0.05 compared with controls.
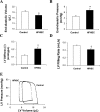
Fig. 2.
Cardiac lysyl oxidase (LOX) gene and protein expression and activity. A: relative mRNA expression of LOX, LOXL-3, and bone morphogenetic protein-1 (BMP-1) as determined by real-time PCR. BMP-1 gene expression was upregulated 1.8-fold after 6 mo of treatment with HFHSC diet. B: Western blot analysis of immunoreactive LOX protein representing the proenzyme (50 kDa) and the mature (30 kDa) forms. C: LOX activity represented by the production of H2O2 and detected by Amplex red oxidation. D: the correlation between cardiac LOX enzymatic activity and LV stiffness (β). Values are presented as means ± SE; n = 3 to 4 mice/group. *P <0.05 compared with controls.
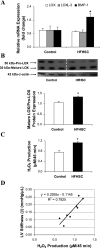
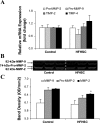
Fig. 3.
Cardiac matrix metalloproteinase (MMP) gene expression and activity. A: relative mRNA expression of pro-MMP-2 and -9, tissue inhibitor of MMP (TIMP)-2 and -4 as determined by real-time PCR. B: representative gelatin zymogram showing changes in MMP-9, pro-MMP-2, and MMP-2 activities. C: the corresponding densitometric analyses of lytic bands. OD, optical density. Values are presented as means ± SE; n = 3 to 4 mice/group. *P <0.01 compared with controls.
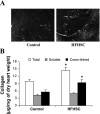
Fig. 4.
Myocardial collagen characteristics. A: Picosirius red image of cardiac tissue. B: myocardial total and cross-linked collagen as determined by hydroxylproline and cyanogen bromide digestion assays. Data are expressed as micrograms of collagen per milligram dry heart weight, assuming that collagen contains an average of 13.5% hydroxyproline. Values are means ± SE; n = 4 mice/group. *P <0.05 compared with controls.
Similar articles
- T lymphocyte regulation of lysyl oxidase in diet-induced cardiac fibrosis.
Zibadi S, Vazquez R, Larson DF, Watson RR. Zibadi S, et al. Cardiovasc Toxicol. 2010 Sep;10(3):190-8. doi: 10.1007/s12012-010-9078-7. Cardiovasc Toxicol. 2010. PMID: 20556665 - Lysyl oxidase overexpression accelerates cardiac remodeling and aggravates angiotensin II-induced hypertrophy.
Galán M, Varona S, Guadall A, Orriols M, Navas M, Aguiló S, de Diego A, Navarro MA, García-Dorado D, Rodríguez-Sinovas A, Martínez-González J, Rodriguez C. Galán M, et al. FASEB J. 2017 Sep;31(9):3787-3799. doi: 10.1096/fj.201601157RR. Epub 2017 May 18. FASEB J. 2017. PMID: 28522596 - T-lymphocytes mediate left ventricular fibrillar collagen cross-linking and diastolic dysfunction in mice.
Yu Q, Vazquez R, Zabadi S, Watson RR, Larson DF. Yu Q, et al. Matrix Biol. 2010 Jul;29(6):511-8. doi: 10.1016/j.matbio.2010.06.003. Epub 2010 Jun 25. Matrix Biol. 2010. PMID: 20600894 Free PMC article. - Role of the lysyl oxidase enzyme family in cardiac function and disease.
Al-U'datt D, Allen BG, Nattel S. Al-U'datt D, et al. Cardiovasc Res. 2019 Nov 1;115(13):1820-1837. doi: 10.1093/cvr/cvz176. Cardiovasc Res. 2019. PMID: 31504232 Review. - Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects.
López B, González A, Hermida N, Valencia F, de Teresa E, Díez J. López B, et al. Am J Physiol Heart Circ Physiol. 2010 Jul;299(1):H1-9. doi: 10.1152/ajpheart.00335.2010. Epub 2010 May 14. Am J Physiol Heart Circ Physiol. 2010. PMID: 20472764 Review.
Cited by
- Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy.
Calligaris SD, Lecanda M, Solis F, Ezquer M, Gutiérrez J, Brandan E, Leiva A, Sobrevia L, Conget P. Calligaris SD, et al. PLoS One. 2013 Apr 11;8(4):e60931. doi: 10.1371/journal.pone.0060931. Print 2013. PLoS One. 2013. PMID: 23593350 Free PMC article. - Maternal obesity enhances collagen accumulation and cross-linking in skeletal muscle of ovine offspring.
Huang Y, Zhao JX, Yan X, Zhu MJ, Long NM, McCormick RJ, Ford SP, Nathanielsz PW, Du M. Huang Y, et al. PLoS One. 2012;7(2):e31691. doi: 10.1371/journal.pone.0031691. Epub 2012 Feb 14. PLoS One. 2012. PMID: 22348119 Free PMC article. - N-acetyl-seryl-aspartyl-lysyl-proline reduces cardiac collagen cross-linking and inflammation in angiotensin II-induced hypertensive rats.
González GE, Rhaleb NE, Nakagawa P, Liao TD, Liu Y, Leung P, Dai X, Yang XP, Carretero OA. González GE, et al. Clin Sci (Lond). 2014 Jan 1;126(1):85-94. doi: 10.1042/CS20120619. Clin Sci (Lond). 2014. PMID: 23834332 Free PMC article. - Cardiac fibrosis.
Frangogiannis NG. Frangogiannis NG. Cardiovasc Res. 2021 May 25;117(6):1450-1488. doi: 10.1093/cvr/cvaa324. Cardiovasc Res. 2021. PMID: 33135058 Free PMC article. Review. - Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities.
Russo I, Frangogiannis NG. Russo I, et al. J Mol Cell Cardiol. 2016 Jan;90:84-93. doi: 10.1016/j.yjmcc.2015.12.011. Epub 2015 Dec 15. J Mol Cell Cardiol. 2016. PMID: 26705059 Free PMC article. Review.
References
- Aubin MC, Lajoie C, Clement R, Gosselin H, Calderone A, Perrault LP. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J Pharmacol Exp Ther 325: 961–968, 2008. - PubMed
- Badenhorst D, Maseko M, Tsotetsi OJ, Naidoo A, Brooksbank R, Norton GR, Woodiwiss AJ. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res 57: 632–641, 2003. - PubMed
- Burlew BS, Weber KT. Connective tissue and the heart. functional significance and regulatory mechanisms. Cardiol Clin 18: 435–442, 2000. - PubMed
- Camera A, Hopps E, Caimi G. Metabolic syndrome: from insulin resistance to adipose tissue dysfunction. Minerva Med 99: 307–321, 2008. - PubMed
- Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick HE, Lee ET, Best LG, de Simone G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (The Strong Heart study). Am J Cardiol 93: 40–44, 2004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials