Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza a viruses - PubMed (original) (raw)
Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza a viruses
Bin Zhou et al. J Virol. 2009 Oct.
Abstract
Pandemic influenza A viruses that emerge from animal reservoirs are inevitable. Therefore, rapid genomic analysis and creation of vaccines are vital. We developed a multisegment reverse transcription-PCR (M-RTPCR) approach that simultaneously amplifies eight genomic RNA segments, irrespective of virus subtype. M-RTPCR amplicons can be used for high-throughput sequencing and/or cloned into modified reverse-genetics plasmids via regions of sequence identity. We used these procedures to rescue a contemporary H3N2 virus and a swine origin H1N1 virus directly from human swab specimens. Together, M-RTPCR and the modified reverse-genetics plasmids that we designed streamline the creation of vaccine seed stocks (9 to 12 days).
Figures
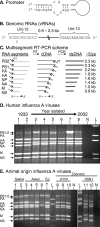
FIG. 1.
M-RTPCR designed to exploit the conserved elements of the influenza A virus promoter. (A) Classical panhandle depiction of base pairing between the 3′ and 5′ termini that occurs in each of the genomic RNA segments. (B) Illustration of genomic RNA segments (vRNAs) of influenza A virus. (C) M-RTPCR scheme illustrating the tailed reverse transcription primer P1, which is complementary to the Uni-12 sequence (e.g., MBTuni-12), and the tailed forward primer P2, which is complementary to the Uni-13 sequence (e.g., MBTuni-13). dsDNA, double-stranded DNA. (D) M-RTPCR successfully amplifies genomes from pandemic human influenza A viruses isolated from 1933 to 2002, regardless of subtype. The influenza A virus strains used were (i) A/WS/1933(H1N1), (ii) A/PR/8/1934(H1N1), (iii) A/Weiss/1947(H1N1), (iv) A/Denver/1/1957(H1N1), (v) A/Japan/305/1957(H2N2), (vi) A/HK/8/1968(H3N2), (vii) A/USSR/1977(H1N1), and (viii) A/NY/1469/2002(H3N2). N, negative control; L DNA ladder (1 kb plus; Invitrogen). (E) Genomic amplification of multiple subtypes of influenza A viruses isolated from swine, avian, equine, and zoonotic viruses that caused fatalities in humans. The influenza A viruses used were (i) A/Sw/IN/1728/1988(H1N1), (ii) A/Sw/WI/1915/1988(H1N1), (iii) A/Dk/Alb/35/1976(H1N1), (iv) A/Mal/WI/944/1982(H5N2), (v) A/R26(Ty/Ont/7732/1966-WSN/1933(H1N9)), (vi) A/Ty/Ont/7732/1966(H5N9), (vii) A/Eq2/Mia/1/1963(H3N8), (viii) A/NJ/8/1976(H1N1), (ix) A/WI/3523/1988(H1N1), (x) A/MD/12/1991(H1N1), (xi) A/HK/156/1997(H5N1), and (xii) A/HK/213/2003(H5N1). N, no-template M-RTPCR control; L, DNA ladder (1 kb plus). For panels D and E, arrows indicating each of the vRNA segments amplified are on the left; note that it is possible to differentiate the PA segment (2.2 kb) from the PB1 and PB2 segments (2.3 kb) for most of the strains. Amplicons were electrophoresed through a 1.5% agarose gel and stained with ethidium bromide.
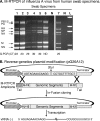
FIG. 2.
Genomic amplification of human influenza A viruses directly from clinical swab specimens. (A). M-RTPCR of influenza A virus rescued directly from human swab specimens. RNA was isolated directly from nas and oro swab specimens collected in New York during the 2005/2006 influenza season (lanes 1 to 5), the 2001/2002 season (lane 6), and the 2008/2009 season that had previously tested positive for influenza A virus. Work with blinded patient swab specimens was done in accordance with Institutional Review Board protocols 07-022 and 09-025. The vRNA was isolated from 100 μl of swab suspension, and one-sixth of the total RNA was amplified by M-RTPCR and subjected to agarose gel electrophoresis. The amplicons shown are from the (i) H3N2 (nas), (ii) H3N2 (oro), (iii) H3N2 (nas or oro), (iv) H3N2 (nas), (v) H3N2 (oro), (vi) H1N1 (nas), and (vii) swine origin H1N1 (nas) subtypes. Where known, the real-time RT-PCR CT corresponding to a particular swab is listed below the lanes. ND, not determined; N, no-template M-RTPCR control; and L, DNA ladder (1 kb plus; Invitrogen). Amplicons were electrophoresed through a 1.5% agarose gel, which was then stained with ethidium bromide. (B) Modification of reverse-genetics plasmid for in-fusion (Clontech) cloning. Shown is a schematic diagram for modified reverse-genetics vector pG26A12 and cloning of M-RTPCR amplicons for generation of vRNA expression plasmids. Twenty-five nucleotides, comprising the 13 nucleotides of the 5′ termini (Uni13) and the 12 nucleotides of the 3′ termini (Uni12) of all influenza A vRNAs, were inserted between the RNA polymerase 1 promoter and terminator. pG26A12 is linearized by StuI digestion, exposing nucleotides identical to those at the termini of M-RTPCR amplicons. The M-RTPCR amplicons have the same termini (Uni13/Uni12 plus some sequence of the Pol I promoter/terminator) as the linearized plasmid for facilitating in-fusion cloning. The clones generated produce authentic vRNA upon transfection into appropriate cells.
FIG. 3.
Reverse-genetics-rescued A/New York/238/05(H3N2) (rgNY238) has a plaque phenotype similar to and growth kinetics equivalent to those for the cell culture-isolated virus (wtNY238). (A) Plaque phenotypes of the rgNY238 and wtNY238 viruses on MDCK cells with an agarose overlay. Representative pictures were taken at 4 days postinfection. (B) Multistep growth curves of rgNY238 and wtNY238 viruses. Confluent MDCK cells were inoculated at a multiplicity of infection of 0.02 TCID50/cell. At 1 hour postinoculation, cells were washed three times with phosphate-buffered saline, and then maintenance medium with tosylsulfonyl phenylalanyl chloromethyl ketone-treated trypsin was added. Supernatants were harvested at 1, 2, 7, 14, 21, 28, 35, 42, and 49 h postinoculation and titrated by a TCID50 assay. Data shown are averages of results from three experiments.
FIG. 4.
Illustration of a streamlined scheme for rapid generation of recombinant influenza A viruses that could be used as vaccine seed stocks. The illustration begins with a swab specimen containing any influenza A virus.
Similar articles
- Influenza A virus molecular virology techniques.
Zhou B, Wentworth DE. Zhou B, et al. Methods Mol Biol. 2012;865:175-92. doi: 10.1007/978-1-61779-621-0_11. Methods Mol Biol. 2012. PMID: 22528160 - [Genome sequence analysis of an H3N2 subtype swine influenza virus isolated from Guangdong province in China].
Yao Y, Zhang GH, Liu WJ, Chen TQ, Sun L. Yao Y, et al. Wei Sheng Wu Xue Bao. 2007 Oct;47(5):805-9. Wei Sheng Wu Xue Bao. 2007. PMID: 18062253 Chinese. - The genetic match between vaccine strains and circulating seasonal influenza A viruses in Vietnam, 2001-2009.
Vuong CD, Hoang PM, Nguyen HL, Nguyen HT, Nguyen TC, Le TT, Dennis DT, Kapella BK, Kile JC, Le MQ. Vuong CD, et al. Influenza Other Respir Viruses. 2013 Nov;7(6):1151-7. doi: 10.1111/irv.12038. Epub 2012 Nov 8. Influenza Other Respir Viruses. 2013. PMID: 23137010 Free PMC article. - Amplification of the entire genome of influenza A virus H1N1 and H3N2 subtypes by reverse-transcription polymerase chain reaction.
Chan CH, Lin KL, Chan Y, Wang YL, Chi YT, Tu HL, Shieh HK, Liu WT. Chan CH, et al. J Virol Methods. 2006 Sep;136(1-2):38-43. doi: 10.1016/j.jviromet.2006.03.027. Epub 2006 May 9. J Virol Methods. 2006. PMID: 16687177 - [Swine influenza virus: evolution mechanism and epidemic characterization--a review].
Qi X, Lu C. Qi X, et al. Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
Cited by
- Phylogeography of Influenza A(H3N2) Virus in Peru, 2010-2012.
Pollett S, Nelson MI, Kasper M, Tinoco Y, Simons M, Romero C, Silva M, Lin X, Halpin RA, Fedorova N, Stockwell TB, Wentworth D, Holmes EC, Bausch DG. Pollett S, et al. Emerg Infect Dis. 2015 Aug;21(8):1330-8. doi: 10.3201/eid2108.150084. Emerg Infect Dis. 2015. PMID: 26196599 Free PMC article. - Multiple introductions of avian influenza viruses (H5N1), Laos, 2009-2010.
Sonnberg S, Phommachanh P, Naipospos TS, McKenzie J, Chanthavisouk C, Pathammavong S, Darnell D, Meeduangchanh P, Rubrum AM, Souriya M, Khambounheuang B, Webby RJ, Douangngeun B, Webster RG. Sonnberg S, et al. Emerg Infect Dis. 2012 Jul;18(7):1139-43. doi: 10.3201/eid1807.111642. Emerg Infect Dis. 2012. PMID: 22710372 Free PMC article. - Swine Influenza Virus PA and Neuraminidase Gene Reassortment into Human H1N1 Influenza Virus Is Associated with an Altered Pathogenic Phenotype Linked to Increased MIP-2 Expression.
Dlugolenski D, Jones L, Howerth E, Wentworth D, Tompkins SM, Tripp RA. Dlugolenski D, et al. J Virol. 2015 May;89(10):5651-67. doi: 10.1128/JVI.00087-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762737 Free PMC article. - Mass vaccination with reassortment-impaired live H9N2 avian influenza vaccine.
Cargnin Faccin F, Cáceres CJ, Gay LC, Seibert B, van Bentem N, Rodriguez LA, Soares Fraiha AL, Cardenas M, Geiger G, Ortiz L, Carnaccini S, Kapczynski DR, Rajao DS, Perez DR. Cargnin Faccin F, et al. NPJ Vaccines. 2024 Aug 3;9(1):136. doi: 10.1038/s41541-024-00923-y. NPJ Vaccines. 2024. PMID: 39097573 Free PMC article. - Phylogenetic and genetic characterization of influenza A H9N2 viruses isolated from backyard poultry in selected farms in Ghana.
Kotey EN, Asante IA, Adusei-Poku M, Arjarquah A, Ampadu R, Rodgers D, Nyarko EO, Asiedu W, Dafeamekpor C, Wiley MR, Mawuli G, Obeng RA, Nyarko SO, Magnusen V, Kodua E, Attram N, Nimo-Paintsil SC, Pratt C, Fox AT, Letizia A, Ampofo WK. Kotey EN, et al. Vet Med Sci. 2022 Jul;8(4):1570-1577. doi: 10.1002/vms3.809. Epub 2022 Apr 21. Vet Med Sci. 2022. PMID: 35451231 Free PMC article.
References
- Adeyefa, C. A., K. Quayle, and J. W. McCauley. 1994. A rapid method for the analysis of influenza virus genes: application to the reassortment of equine influenza virus genes. Virus Res. 32:391-399. - PubMed
- Chan, C. H., K. L. Lin, Y. Chan, Y. L. Wang, Y. T. Chi, H. L. Tu, H. K. Shieh, and W. T. Liu. 2006. Amplification of the entire genome of influenza A virus H1N1 and H3N2 subtypes by reverse-transcription polymerase chain reaction. J. Virol. Methods 136:38-43. - PubMed
- de Wit, E., T. M. Bestebroer, M. I. Spronken, G. F. Rimmelzwaan, A. D. Osterhaus, and R. A. Fouchier. 2007. Rapid sequencing of the non-coding regions of influenza A virus. J. Virol. Methods 139:85-89. - PubMed
- Flick, R., and G. Hobom. 1999. Interaction of influenza virus polymerase with viral RNA in the ‘corkscrew’ conformation. J. Gen. Virol. 80:2565-2572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical