Genome-wide identification of Saccharomyces cerevisiae genes required for maximal tolerance to ethanol - PubMed (original) (raw)
Genome-wide identification of Saccharomyces cerevisiae genes required for maximal tolerance to ethanol
Miguel C Teixeira et al. Appl Environ Microbiol. 2009 Sep.
Abstract
The understanding of the molecular basis of yeast resistance to ethanol may guide the design of rational strategies to increase process performance in industrial alcoholic fermentations. In this study, the yeast disruptome was screened for mutants with differential susceptibility to stress induced by high ethanol concentrations in minimal growth medium. Over 250 determinants of resistance to ethanol were identified. The most significant gene ontology terms enriched in this data set are those associated with intracellular organization, biogenesis, and transport, in particular, regarding the vacuole, the peroxisome, the endosome, and the cytoskeleton, and those associated with the transcriptional machinery. Clustering the proteins encoded by the identified determinants of ethanol resistance by their known physical and genetic interactions highlighted the importance of the vacuolar protein sorting machinery, the vacuolar H(+)-ATPase complex, and the peroxisome protein import machinery. Evidence showing that vacuolar acidification and increased resistance to the cell wall lytic enzyme beta-glucanase occur in response to ethanol-induced stress was obtained. Based on the genome-wide results, the particular role of the FPS1 gene, encoding a plasma membrane aquaglyceroporin which mediates controlled glycerol efflux, in ethanol stress resistance was further investigated. FPS1 expression contributes to decreased [(3)H]ethanol accumulation in yeast cells, suggesting that Fps1p may also play a role in maintaining the intracellular ethanol level during active fermentation. The increased expression of FPS1 confirmed the important role of this gene in alcoholic fermentation, leading to increased final ethanol concentration under conditions that lead to high ethanol production.
Figures
FIG. 1.
Categorization, based on the biological process taxonomy of GO, of the genes required for ethanol stress resistance. Genes were clustered using GOToolBox, and only classes found to be statistically overrepresented in our data set are displayed (P value below 0.01). Striped bars, gene frequency within each class; black bars, frequency registered for the whole genome.
FIG. 2.
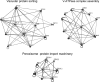
Main interaction network maps of the determinants of ethanol tolerance. The map was created using the OSPREY software and shows protein and genetic interactions within three groups of genes associated with vacuolar protein sorting, V-ATPase complex assembly, and peroxisome protein import machinery. The existence of an interaction between two genes or proteins is represented by a connection between two nodes.
FIG. 3.
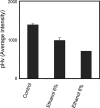
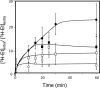
pHv, assessed as fluorescence intensity values as described in Materials and Methods, upon 1 h of BY4741 cell cultivation in growth medium in the absence (control) or presence of inhibitory concentrations of ethanol (6% or 8% [vol/vol]). Values are the averages of at least three independent experiments; standard variations are displayed as error bars.
FIG. 4.
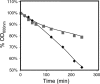
Comparison of the susceptibilities to lyticase of cells of S. cerevisiae parental strain BY4741 grown in the absence of ethanol and then incubated in a growth medium that was either left unsupplemented (⧫) or supplemented with 6% ethanol (▪). Cells were harvested after 3 h of incubation. The different cell populations were washed with water and resuspended in 0.1 M sodium phosphate buffer at pH 7.5. After the addition of 20 μg of lyticase (Sigma) per ml, the decrease in the OD600 of the cell suspension was measured periodically.
FIG. 5.
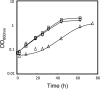
Comparison of the susceptibilities to ethanol-induced stress of S. cerevisiae parental strain W303-1A, harboring the FPS1 expression plasmid (□) or the corresponding empty vector (⋄), to those of the derived deletion mutant Δ_fps1_, harboring the FPS1 expression plasmid (○) or the corresponding empty vector (▵). Cells were grown in the absence of ethanol and then incubated in MM4 liquid medium supplemented with 6% ethanol. Growth curves are representative of at least three independent experiments.
FIG. 6.
Comparison of levels of [3H]ethanol accumulation in nonadapted cells of S. cerevisiae parental strain W303-1A, harboring the FPS1 expression plasmid (○) or the corresponding empty vector (□), and the derived Δ_fps1_ deletion mutant, harboring the FPS1 expression plasmid (▪) or the corresponding empty vector (•), during cultivation in MM4 liquid medium suddenly supplemented with 6% ethanol under conditions identical to those used for Fig. 5. The accumulation values are representative of at least three independent experiments. [3H-Et]intra and [3H-Et]extra, intracellular and extracellular [3H]ethanol concentrations, respectively.
FIG. 7.
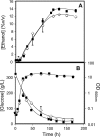
Comparison of extracellular concentrations of ethanol and glucose, accumulated during cultivation in fermentation medium of BY4741 yeast cells harboring the pYEP-FPS1 plasmid (▪) or the corresponding cloning vector (⋄). Growth was followed by measuring culture OD600.
Similar articles
- Vacuolar H+-ATPase Protects Saccharomyces cerevisiae Cells against Ethanol-Induced Oxidative and Cell Wall Stresses.
Charoenbhakdi S, Dokpikul T, Burphan T, Techo T, Auesukaree C. Charoenbhakdi S, et al. Appl Environ Microbiol. 2016 May 2;82(10):3121-3130. doi: 10.1128/AEM.00376-16. Print 2016 May 15. Appl Environ Microbiol. 2016. PMID: 26994074 Free PMC article. - Increased expression of the yeast multidrug resistance ABC transporter Pdr18 leads to increased ethanol tolerance and ethanol production in high gravity alcoholic fermentation.
Teixeira MC, Godinho CP, Cabrito TR, Mira NP, Sá-Correia I. Teixeira MC, et al. Microb Cell Fact. 2012 Jul 27;11:98. doi: 10.1186/1475-2859-11-98. Microb Cell Fact. 2012. PMID: 22839110 Free PMC article. - The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae.
Stanley D, Bandara A, Fraser S, Chambers PJ, Stanley GA. Stanley D, et al. J Appl Microbiol. 2010 Jul;109(1):13-24. doi: 10.1111/j.1365-2672.2009.04657.x. Epub 2010 Jan 11. J Appl Microbiol. 2010. PMID: 20070446 Review. - Omics analysis of acetic acid tolerance in Saccharomyces cerevisiae.
Geng P, Zhang L, Shi GY. Geng P, et al. World J Microbiol Biotechnol. 2017 May;33(5):94. doi: 10.1007/s11274-017-2259-9. Epub 2017 Apr 12. World J Microbiol Biotechnol. 2017. PMID: 28405910 Review.
Cited by
- Protective effects of peptides on the cell wall structure of yeast under osmotic stress.
Jin X, Chen M, Coldea TE, Yang H, Zhao H. Jin X, et al. Appl Microbiol Biotechnol. 2022 Nov;106(21):7051-7061. doi: 10.1007/s00253-022-12207-3. Epub 2022 Oct 3. Appl Microbiol Biotechnol. 2022. PMID: 36184688 - Physiology of yeast strains isolated from Brazilian biomes in a minimal medium using fructose as the sole carbon source reveals potential biotechnological applications.
de Andrade Silva CA, Oka ML, Fonseca GG. de Andrade Silva CA, et al. 3 Biotech. 2019 May;9(5):191. doi: 10.1007/s13205-019-1721-9. Epub 2019 Apr 26. 3 Biotech. 2019. PMID: 31065491 Free PMC article. - An evolutionarily conserved mechanism of calcium-dependent neurotoxicity in a zebrafish model of fetal alcohol spectrum disorders.
Flentke GR, Klingler RH, Tanguay RL, Carvan MJ 3rd, Smith SM. Flentke GR, et al. Alcohol Clin Exp Res. 2014 May;38(5):1255-65. doi: 10.1111/acer.12360. Epub 2014 Feb 11. Alcohol Clin Exp Res. 2014. PMID: 24512079 Free PMC article. - Ethanol Cellular Defense Induce Unfolded Protein Response in Yeast.
Navarro-Tapia E, Nana RK, Querol A, Pérez-Torrado R. Navarro-Tapia E, et al. Front Microbiol. 2016 Feb 18;7:189. doi: 10.3389/fmicb.2016.00189. eCollection 2016. Front Microbiol. 2016. PMID: 26925053 Free PMC article. - Shared and more specific genetic determinants and pathways underlying yeast tolerance to acetic, butyric, and octanoic acids.
Mota MN, Matos M, Bahri N, Sá-Correia I. Mota MN, et al. Microb Cell Fact. 2024 Feb 29;23(1):71. doi: 10.1186/s12934-024-02309-0. Microb Cell Fact. 2024. PMID: 38419072 Free PMC article.
References
- Aguilera, F., R. A. Peinado, C. Millan, J. M. Ortega, and J. C. Mauricio. 2006. Relationship between ethanol tolerance, H+-ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int. J. Food Microbiol. 110:34-42. - PubMed
- Alexandre, H., V. Ansanay-Galeote, S. Dequin, and B. Blondin. 2001. Global gene expression during short-term ethanol stress in Saccharomyces cerevisiae. FEBS Lett. 498:98-103. - PubMed
- Alper, H., J. Moxley, E. Nevoigt, G. R. Fink, and G. Stephanopoulos. 2006. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science 314:1565-1568. - PubMed
- Carmelo, V., H. Santos, and I. Sá-Correia. 1997. Effect of extracellular acidification on the activity of plasma membrane ATPase and on the cytosolic and vacuolar pH of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1325:63-70. - PubMed
- Casey, G. P., and W. M. Ingledew. 1986. Ethanol tolerance in yeasts. Crit. Rev. Microbiol. 13:219-280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases