Atorvastatin extends the therapeutic window for tPA to 6 h after the onset of embolic stroke in rats - PubMed (original) (raw)
Atorvastatin extends the therapeutic window for tPA to 6 h after the onset of embolic stroke in rats
Li Zhang et al. J Cereb Blood Flow Metab. 2009 Nov.
Abstract
We investigated the neuroprotective effect of atorvastatin in combination with delayed thrombolytic therapy in a rat model of embolic stroke. Rats subjected to embolic middle cerebral artery (MCA) occlusion were treated with atorvastatin at 4 h, followed by tissue plasminogen activator (tPA) at 6 or 8 h after stroke. The combination of atorvastatin at 4 h and tPA at 6 h significantly decreased the size of the embolus at the origin of the MCA, improved microvascular patency, and reduced infarct volume, but did not increase the incidence of hemorrhagic transformation compared with vehicle-treated control animals. However, monotherapy with tPA at 6 h increased the incidence of hemorrhagic transformation and failed to reduce infarct volume compared with the control group. In addition, adjuvant treatment with atorvastatin at 4 h and with tPA at 6 h reduced tPA-induced upregulation of protease-activated receptor-1, intercellular adhesion molecule-1, and matrix metalloproteinase-9, and concomitantly reduced cerebral microvascular platelet, neutrophil, and fibrin deposition compared with rats treated with tPA alone at 6 h. In conclusion, a combination of atorvastatin and tPA extended the therapeutic window for stroke to 6 h without increasing the incidence of hemorrhagic transformation. Atorvastatin blocked delayed tPA-potentiated adverse cerebral vascular events, which likely contributes to the neuroprotective effect of the combination therapy.
Figures
Figure 1
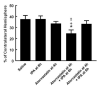
Infarct volume. Bar graph shows the effects of monotherapy with tPA and atorvastatin and the combination therapy on infarct volume assessed 7 days after MCA occlusion. Values are mean ± s.e. *P < 0.05 as compared with that of the saline-treated group. †P < 0.05 as compared with that of the tPA alone group. ‡P < 0.05 as compared with that of the atorvastatin alone group.
Figure 2
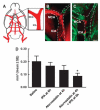
Embolus at the origin of the occluded MCA. (A) Schematic representation of a ventral view of a rat brain is shown. The enlarged boxed area from panel A shows the intracranial segment of the ICA and the MCA. A large fragment of Evans blue dye (B, red) was detected within the origin of the MCA and intracranial segment of the ICA, which blocked plasma perfusion (panel B, green) from a representative rat treated with tPA alone. The combination treatment with atorvastatin at 4 h and tPA at 6 h resulted in only a small fragment of Evans blue dye (C, red) at the origin of the MCA and the intracranial segment of the ICA, which were perfused by FITC-dextran (panel C, green). (D) shows the quantitative data of an embolus (_n_=4 per group). Bar in panels B and C=400 _μ_m.
Figure 3
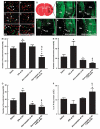
Thrombocyte, fibrin/fibrinogen, MPO, ICAM-1, MMP9, and collagen type IV immunoreactive cerebral vessels and FITC-dextran perfusion. Double immunofluorescent staining (A–F) shows thrombocyte (panels A and B, green), fibrin/fibrinogen (panels C and D, green), and MPO (panels E and F, green) in cerebral blood vessels (Eba, panels A–F, red) of representative rats treated with tPA alone (panels A, C, and E) and with a combination of atorvastatin at 4 h and tPA at 6 h (panels B, D, and F). (G–L) The cerebral microvessels perfused with FITC-dextran in a normal rat (panel H), and in representative rats treated with saline (panel I), tPA (panel J), atorvastatin (panel K), and a combination of tPA at 4 h and atorvastatin at 6 h (panel L) 30 h after MCA occlusion (n_=4 per group) is shown. (M–P) Represents the quantitative data of the number of thrombocyte (panel M), fibrin/fibrinogen (panel N), MPO immunoreactive cerebral vessels (panel O), and microvessels perfused with FITC-dextran (panel P). Double immunostaining shows that cerebral blood vessels with diffused collagen type IV immunoreactivity (Q, R, T, U; red) were ICAM-1 (panels Q and R, green) or MMP9 positive (panels T and U, green) in representative rats treated with tPA alone (panels Q and T), and with a combination of atorvastatin at 4 h and tPA at 6 h (panels R and U). Panel S and (V) show intensive collagen type IV immunoreactivity (red) in contralateral homologous areas wherein ICAM-1 (panel C, green) and MMP9 (panel F, green) were absent from a representative rat treated with tPA alone. Double immunostaining (W–Y) shows that ICAM-1 (panels W and Y, green; arrows) and MMP9 (panels X and Y, brown; arrowheads) immunoreactivities were in different vessels of a representative rat treated with tPA alone. Panel Y is a merged image. Ctx, cortex; Str, striatum; CC, corpus callosum. *P < 0.05 as compared with that of saline-treated group. †_P<0.05 as compared with that of the tPA alone group. ‡P<0.05 as compared with that of the atorvastatin alone group. §P<0.05 for treatment interaction (synergistic effect). Bars for panels A–F=40 _μ_m; panels H–L=1 mm; panels Q–Y=40 _μ_m.
Figure 3
Thrombocyte, fibrin/fibrinogen, MPO, ICAM-1, MMP9, and collagen type IV immunoreactive cerebral vessels and FITC-dextran perfusion. Double immunofluorescent staining (A–F) shows thrombocyte (panels A and B, green), fibrin/fibrinogen (panels C and D, green), and MPO (panels E and F, green) in cerebral blood vessels (Eba, panels A–F, red) of representative rats treated with tPA alone (panels A, C, and E) and with a combination of atorvastatin at 4 h and tPA at 6 h (panels B, D, and F). (G–L) The cerebral microvessels perfused with FITC-dextran in a normal rat (panel H), and in representative rats treated with saline (panel I), tPA (panel J), atorvastatin (panel K), and a combination of tPA at 4 h and atorvastatin at 6 h (panel L) 30 h after MCA occlusion (n_=4 per group) is shown. (M–P) Represents the quantitative data of the number of thrombocyte (panel M), fibrin/fibrinogen (panel N), MPO immunoreactive cerebral vessels (panel O), and microvessels perfused with FITC-dextran (panel P). Double immunostaining shows that cerebral blood vessels with diffused collagen type IV immunoreactivity (Q, R, T, U; red) were ICAM-1 (panels Q and R, green) or MMP9 positive (panels T and U, green) in representative rats treated with tPA alone (panels Q and T), and with a combination of atorvastatin at 4 h and tPA at 6 h (panels R and U). Panel S and (V) show intensive collagen type IV immunoreactivity (red) in contralateral homologous areas wherein ICAM-1 (panel C, green) and MMP9 (panel F, green) were absent from a representative rat treated with tPA alone. Double immunostaining (W–Y) shows that ICAM-1 (panels W and Y, green; arrows) and MMP9 (panels X and Y, brown; arrowheads) immunoreactivities were in different vessels of a representative rat treated with tPA alone. Panel Y is a merged image. Ctx, cortex; Str, striatum; CC, corpus callosum. *P < 0.05 as compared with that of saline-treated group. †_P<0.05 as compared with that of the tPA alone group. ‡P<0.05 as compared with that of the atorvastatin alone group. §P<0.05 for treatment interaction (synergistic effect). Bars for panels A–F=40 _μ_m; panels H–L=1 mm; panels Q–Y=40 _μ_m.
Similar articles
- Adjuvant treatment with a glycoprotein IIb/IIIa receptor inhibitor increases the therapeutic window for low-dose tissue plasminogen activator administration in a rat model of embolic stroke.
Zhang L, Zhang ZG, Zhang R, Morris D, Lu M, Coller BS, Chopp M. Zhang L, et al. Circulation. 2003 Jun 10;107(22):2837-43. doi: 10.1161/01.CIR.0000068374.57764.EB. Epub 2003 May 19. Circulation. 2003. PMID: 12756151 - Transient brain hypothermia reduces the reperfusion injury of delayed tissue plasminogen activator and extends its therapeutic time window in a focal embolic stroke model.
Zarisfi M, Allahtavakoli F, Hassanipour M, Khaksari M, Rezazadeh H, Allahtavakoli M, Taghavi MM. Zarisfi M, et al. Brain Res Bull. 2017 Sep;134:85-90. doi: 10.1016/j.brainresbull.2017.07.007. Epub 2017 Jul 11. Brain Res Bull. 2017. PMID: 28710023 - Intravenous administration of a GPIIb/IIIa receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model.
Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Zhang L, et al. Stroke. 2004 Dec;35(12):2890-5. doi: 10.1161/01.STR.0000147963.68238.da. Epub 2004 Oct 28. Stroke. 2004. PMID: 15514182 - The neurovascular unit and combination treatment strategies for stroke.
Zhang L, Zhang ZG, Chopp M. Zhang L, et al. Trends Pharmacol Sci. 2012 Aug;33(8):415-22. doi: 10.1016/j.tips.2012.04.006. Epub 2012 May 16. Trends Pharmacol Sci. 2012. PMID: 22595494 Free PMC article. Review. - Mmp-9 inhibition: a therapeutic strategy in ischemic stroke.
Chaturvedi M, Kaczmarek L. Chaturvedi M, et al. Mol Neurobiol. 2014 Feb;49(1):563-73. doi: 10.1007/s12035-013-8538-z. Epub 2013 Sep 12. Mol Neurobiol. 2014. PMID: 24026771 Free PMC article. Review.
Cited by
- Development and Testing of Thrombolytics in Stroke.
Nikitin D, Choi S, Mican J, Toul M, Ryu WS, Damborsky J, Mikulik R, Kim DE. Nikitin D, et al. J Stroke. 2021 Jan;23(1):12-36. doi: 10.5853/jos.2020.03349. Epub 2021 Jan 31. J Stroke. 2021. PMID: 33600700 Free PMC article. Review. - Embolic occlusion of internal carotid artery in conscious rats: Immediate effects of cerebral ischemia.
Toung TJK, Mehr N, Mirski M, Koehler RC. Toung TJK, et al. Physiol Rep. 2023 Feb;11(4):e15613. doi: 10.14814/phy2.15613. Physiol Rep. 2023. PMID: 36802121 Free PMC article. - Potential nanotherapeutic strategies for perioperative stroke.
An J, Zhao L, Duan R, Sun K, Lu W, Yang J, Liang Y, Liu J, Zhang Z, Li L, Shi J. An J, et al. CNS Neurosci Ther. 2022 Apr;28(4):510-520. doi: 10.1111/cns.13819. Epub 2022 Mar 4. CNS Neurosci Ther. 2022. PMID: 35243774 Free PMC article. Review. - Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats.
Kanazawa M, Miura M, Toriyabe M, Koyama M, Hatakeyama M, Ishikawa M, Nakajima T, Onodera O, Takahashi T, Nishizawa M, Shimohata T. Kanazawa M, et al. Sci Rep. 2017 Feb 14;7:42582. doi: 10.1038/srep42582. Sci Rep. 2017. PMID: 28195185 Free PMC article. - Differences in Acute Expression of Matrix Metalloproteinases-9, 3, and 2 Related to the Duration of Brain Ischemia and Tissue Plasminogen Activator Treatment in Experimental Stroke.
Wang D, Saleem S, Sullivan RD, Zhao T, Reed GL. Wang D, et al. Int J Mol Sci. 2024 Aug 30;25(17):9442. doi: 10.3390/ijms25179442. Int J Mol Sci. 2024. PMID: 39273389 Free PMC article.
References
- Black AE, Hayes RN, Roth BD, Woo P, Woolf TF. Metabolism and excretion of atorvastatin in rats and dogs. Drug Metab Dispos. 1999;27:916–23. - PubMed
- Braaten JV, Jerome WG, Hantgan RR. Uncoupling fibrin from integrin receptors hastens fibrinolysis at the platelet-fibrin interface. Blood. 1994;83:982–93. - PubMed
- Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Davalos A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS023393/NS/NINDS NIH HHS/United States
- R01 HL064766/HL/NHLBI NIH HHS/United States
- R01 HL64766/HL/NHLBI NIH HHS/United States
- P01 NS023393-18/NS/NINDS NIH HHS/United States
- P01 NS23393/NS/NINDS NIH HHS/United States
- R01 HL064766-08/HL/NHLBI NIH HHS/United States
- R01 NS062832-01/NS/NINDS NIH HHS/United States
- R01 NS062832/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical