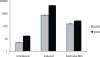A retro-lentiviral system for doxycycline-inducible gene expression and gene knockdown in cells with limited proliferative capacity - PubMed (original) (raw)
A retro-lentiviral system for doxycycline-inducible gene expression and gene knockdown in cells with limited proliferative capacity
Mikhail F Alexeyev et al. Mol Biol Rep. 2010 Apr.
Abstract
Currently, there is no reliable system for regulated gene expression and regulated gene knockdown in cells with finite lifespan. In this manuscript, we describe a vector system, consisting of a retrovirus for the delivery of rtTA, and a lentivirus for the delivery of either a transgene or a miR-shRNA for the modification of primary cells. Primary rat pulmonary microvascular endothelial cells (PMVEC) modified by these vectors for the inducible expression of Gaussia luciferase or DsRed Express demonstrated greater than 100-fold induction of the transgene expression with doxycycline. The system works reliably in both sequential and simultaneous infection modes, with about 95% of the sells selected with two antibiotics being inducible in each mode. The lentiviral vector for gene knockdown allows for the direct cloning of shRNA oligos using alpha-complementation, and for the monitoring of induction of RNA interference with fluorescent reporter, mCherry. The gene knockdown vector was validated by knocking down beta-actin expression in PMVECs, with two of the four constructs showing 59 and 75% knockdown, respectively, compared to uninduced controls. The vectors described here were successfully used for the modification of various primary and established cell lines for regulated gene expression and regulated knockdown.
Figures
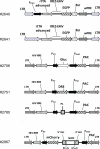
Fig. 1
Vector maps. Abbreviations: 5’ and 3’ mir, 5’ and 3’ flanking sequences derived from the murine mir-155 micro-RNA gene; Bsr, blasticidin resistance gene; DRE, DsRed express; EGFP, enhanced green fluorescent protein; Gluc, (secreted) Gaussia luciferase; HIV RRE, human immunodeficiency virus rev response element; IRES EMV, encephalomyelocarditis virus internal ribosome entry site; lacZα, gene encoding the alpha-fragment of E. coli β-galactosidase gene; LTR, retro/lentiviral long terminal repeat; PAC, puromycin resistance gene; PL, polylinker. Unique sites 5’-EcoRI, XhoI, ApaI, MluI, NotI, XbaI, SpeI, BamHI, SmaI, PstI-3’; PPGK, phosphoglycerate kinase promoter; PCMV, CMV promoter; PSV40, SV40 promoter; PTet, doxycycline-regulated promoter; rtTA, reverse tetracycline-controlled transactivator protein; wPRE, woodchuck hepatitis virus posttranscriptional regulatory element.
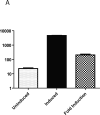
Fig. 2
Luciferase inducibility of PMVECs. PMVECs were infected with retrovirus 2640 or 2641, selected for 5 days with 30 μg/ml blasticidin, infected with lentivirus #2706, and induced with 3 μg/ml of doxycycline for 24h. Luciferase assays were performed as described in Materials and Methods. Luciferase activity is expressed in relative light units. The data are mean ± SEM (n=4).
Fig. 3
Inducibility of DRE and percent responsive PMVECs as reported by FACS. A, inducibility. B, percent responsive cells. PMVECs were simultaneously infected with retrovirus #2641 and lentivirus #2706 overnight, allowed to recover for 24h in DMEM, and selected with 30 μg/ml blasticidin for 2 days followed by selection with 30 μg/ml blasticidin plus 5 μg/ml puromycin for 1 day and finally with blasticidin and puromycin ± 3 μg/ml of doxycycline for 2 days. At the end of experiment, cells were collected by trypsinization and subjected to FACS analysis. The data are mean ± SEM (n=3).
Fig. 3
Inducibility of DRE and percent responsive PMVECs as reported by FACS. A, inducibility. B, percent responsive cells. PMVECs were simultaneously infected with retrovirus #2641 and lentivirus #2706 overnight, allowed to recover for 24h in DMEM, and selected with 30 μg/ml blasticidin for 2 days followed by selection with 30 μg/ml blasticidin plus 5 μg/ml puromycin for 1 day and finally with blasticidin and puromycin ± 3 μg/ml of doxycycline for 2 days. At the end of experiment, cells were collected by trypsinization and subjected to FACS analysis. The data are mean ± SEM (n=3).
Fig. 4
Knockdown of β-actin in PMVECs. For knockdown experiments, four lentiviral constructs were generated, which targeted four regions of the rat β-actin mRNA (target sequences: #1= gaagatttggcaccacacttt, #2=caggctgtgttgtccctgtat, #3= gaaatcgtgcgtgacattaaa, and 4= cctgacagactacctcatgaa). PMVECs were infected first with #2641 (selection for blasticidin resistance, 30 ug/ml, 5 days), and then with one of the knockdown constructs (selected for 3 days with with 5 ug/ml of puromycin). Cells were either induced (+) or not (−) with 3 μg/ml of doxycycline (Dox). Western Blotting was performed as described in Materials and Methods, chemiluminescense was documented with Fujifilm LAS-1000 cooled CCD camera, and analyzed with MultiGauge 3.0 program.
Similar articles
- Lentiviral vector-mediated doxycycline-inducible USP39 shRNA or cDNA expression in triple-negative breast cancer cells.
Liu S, Liu X, Wang H, Zhou Q, Liang Y, Sui A, Yao R, Zhao B, Sun M. Liu S, et al. Oncol Rep. 2015 May;33(5):2477-83. doi: 10.3892/or.2015.3872. Epub 2015 Mar 20. Oncol Rep. 2015. PMID: 25812575 - Doxycycline-dependent inducible and reversible RNA interference mediated by a single lentivirus vector.
Matsushita N, Matsushita S, Hirakawa S, Higashiyama S. Matsushita N, et al. Biosci Biotechnol Biochem. 2013;77(4):776-81. doi: 10.1271/bbb.120917. Epub 2013 Apr 7. Biosci Biotechnol Biochem. 2013. PMID: 23563548 - Doxycycline modulates VEGF-A expression: Failure of doxycycline-inducible lentivirus shRNA vector to knockdown VEGF-A expression in transgenic mice.
Merentie M, Rissanen R, Lottonen-Raikaslehto L, Huusko J, Gurzeler E, Turunen MP, Holappa L, Mäkinen P, Ylä-Herttuala S. Merentie M, et al. PLoS One. 2018 Jan 19;13(1):e0190981. doi: 10.1371/journal.pone.0190981. eCollection 2018. PLoS One. 2018. PMID: 29351307 Free PMC article. - A novel lentivirus for quantitative assessment of gene knockdown in stem cell differentiation.
Alimperti S, Lei P, Tian J, Andreadis ST. Alimperti S, et al. Gene Ther. 2012 Dec;19(12):1123-32. doi: 10.1038/gt.2011.208. Epub 2012 Jan 12. Gene Ther. 2012. PMID: 22241174 Review. - Toward Tightly Tuned Gene Expression Following Lentiviral Vector Transduction.
Page A, Fusil F, Cosset FL. Page A, et al. Viruses. 2020 Dec 11;12(12):1427. doi: 10.3390/v12121427. Viruses. 2020. PMID: 33322556 Free PMC article. Review.
Cited by
- Lactate dehydrogenase a expression is necessary to sustain rapid angiogenesis of pulmonary microvascular endothelium.
Parra-Bonilla G, Alvarez DF, Alexeyev M, Vasauskas A, Stevens T. Parra-Bonilla G, et al. PLoS One. 2013 Sep 26;8(9):e75984. doi: 10.1371/journal.pone.0075984. eCollection 2013. PLoS One. 2013. PMID: 24086675 Free PMC article. - Progesterone receptor (PR) polyproline domain (PPD) mediates inhibition of epidermal growth factor receptor (EGFR) signaling in non-small cell lung cancer cells.
Kawprasertsri S, Pietras RJ, Marquez-Garban DC, Boonyaratanakornkit V. Kawprasertsri S, et al. Cancer Lett. 2016 May 1;374(2):279-91. doi: 10.1016/j.canlet.2016.02.014. Epub 2016 Feb 15. Cancer Lett. 2016. PMID: 26892043 Free PMC article. - Galectin-12 inhibits granulocytic differentiation of human NB4 promyelocytic leukemia cells while promoting lipogenesis.
Xue H, Yang RY, Tai G, Liu FT. Xue H, et al. J Leukoc Biol. 2016 Oct;100(4):657-664. doi: 10.1189/jlb.1HI0316-134R. Epub 2016 Jun 2. J Leukoc Biol. 2016. PMID: 27256573 Free PMC article. - Identification of VPS13C as a Galectin-12-Binding Protein That Regulates Galectin-12 Protein Stability and Adipogenesis.
Yang RY, Xue H, Yu L, Velayos-Baeza A, Monaco AP, Liu FT. Yang RY, et al. PLoS One. 2016 Apr 13;11(4):e0153534. doi: 10.1371/journal.pone.0153534. eCollection 2016. PLoS One. 2016. PMID: 27073999 Free PMC article. - N-cadherin coordinates AMP kinase-mediated lung vascular repair.
Jian MY, Liu Y, Li Q, Wolkowicz P, Alexeyev M, Zmijewski J, Creighton J. Jian MY, et al. Am J Physiol Lung Cell Mol Physiol. 2016 Jan 1;310(1):L71-85. doi: 10.1152/ajplung.00227.2015. Epub 2015 Nov 6. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26545901 Free PMC article.
References
- Pohjoismaki Jl, Wanrooij S, Hyvarinen Ak, et al. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic acids research. 2006;34(20):5815–5828. doi:gkl703 [pii] 10.1093/nar/gkl703. - PMC - PubMed
- Pastukh V, Shokolenko I, Wang B, et al. Human mitochondrial transcription factor A possesses multiple subcellular targeting signals. FEBS J. 2007;274(24):6488–6499. doi:EJB6167 [pii] 10.1111/j.1742-4658.2007.06167.x. - PubMed
- Sambrook J, Russel Dw. A laboratory manual. Cold Spring Harbor Laboratory Press; New York: 2001. Molecular Cloning.
- King J, Hamil T, Creighton J, et al. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67(2):139–151. doi:10.1016/j.mvr.2003.11.006 S002628620300116X [pii] - PubMed
- Rappa G, Anzanello F, Alexeyev M, et al. Gamma-glutamylcysteine synthetase-based selection strategy for gene therapy of chronic granulomatous disease and graft-vs.-host disease. Eur J Haematol. 2007;78(5):440–448. doi:EJH833 [pii] 10.1111/j.1600-0609.2007.00833.x. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 RR023961/RR/NCRR NIH HHS/United States
- P01 HL06629907/HL/NHLBI NIH HHS/United States
- P01 HL066299/HL/NHLBI NIH HHS/United States
- R01 RR031286/RR/NCRR NIH HHS/United States
- R01 OD010944/OD/NIH HHS/United States
- P01 HL066299-079002/HL/NHLBI NIH HHS/United States
- R21 RR023961-01A1/RR/NCRR NIH HHS/United States
- R21RR02396101/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials